Physical Property
... Since chemistry is the study of matter, we begin by defining matter. Matter: anything that has mass and takes up space (has a volume). Anything that is not energy is matter. Mass: a measure of how much matter is present. An element is a substance that can not be broken down into simpler substances. ...
... Since chemistry is the study of matter, we begin by defining matter. Matter: anything that has mass and takes up space (has a volume). Anything that is not energy is matter. Mass: a measure of how much matter is present. An element is a substance that can not be broken down into simpler substances. ...
Chapter 15- Classification of Matter
... i. Substance does not change __________________ when it undergoes a physical change. ii. __________________ is a process for separating a mixture by evaporating a liquid and condensing its vapor. c. _________________________- characteristics of a substance indicating that it can change chemically; f ...
... i. Substance does not change __________________ when it undergoes a physical change. ii. __________________ is a process for separating a mixture by evaporating a liquid and condensing its vapor. c. _________________________- characteristics of a substance indicating that it can change chemically; f ...
Review topics-blog
... rearranging the atoms into new substances in the products. As an example, CH4 (methane) reacts with O2 (oxygen gas) to form carbon dioxide (CO2) and water (H2O). All of the carbon in methane will end as all of the carbon dioxide (CO2). All of the hydrogen in methane ends up in the water molecule ...
... rearranging the atoms into new substances in the products. As an example, CH4 (methane) reacts with O2 (oxygen gas) to form carbon dioxide (CO2) and water (H2O). All of the carbon in methane will end as all of the carbon dioxide (CO2). All of the hydrogen in methane ends up in the water molecule ...
Adv review key
... full shell. Also tell what charge it would have (positive or negative and how much ex: +2) ...
... full shell. Also tell what charge it would have (positive or negative and how much ex: +2) ...
APS 1st semester exam review 2016
... full shell. Also tell what charge it would have (positive or negative and how much ex: +2) ...
... full shell. Also tell what charge it would have (positive or negative and how much ex: +2) ...
Fall - Physical Chemistry Division
... dimensionality starting from 0D fullerenes, to 1D carbon nanotubes (CNTs) and graphene nanoribbons (GNRs), 2D single-layered (or few layered) graphene, up to 3D graphite, their derivatives, and intercalated compounds. Graphitic materials are interesting both from a basic research viewpoint and for i ...
... dimensionality starting from 0D fullerenes, to 1D carbon nanotubes (CNTs) and graphene nanoribbons (GNRs), 2D single-layered (or few layered) graphene, up to 3D graphite, their derivatives, and intercalated compounds. Graphitic materials are interesting both from a basic research viewpoint and for i ...
Introduction to Chemistry
... Matter can be classified as either a gas, liquid, or solid Generally, as you add energy, particles move faster and farther apart As you remove energy, particles move closer and become attracted to each other ...
... Matter can be classified as either a gas, liquid, or solid Generally, as you add energy, particles move faster and farther apart As you remove energy, particles move closer and become attracted to each other ...
key
... Placed in table above using blue electrons. We predict it to be a colorless gas with low electrical conductivity and high electrical reactivity. c) Are there any elements that have not yet been discovered? If so, what would their properties be? This table has room for four more elements. The element ...
... Placed in table above using blue electrons. We predict it to be a colorless gas with low electrical conductivity and high electrical reactivity. c) Are there any elements that have not yet been discovered? If so, what would their properties be? This table has room for four more elements. The element ...
Final Exam Review Guide
... 1. Liquids have the greatest amount of kinetic energy among the phases of matter ...
... 1. Liquids have the greatest amount of kinetic energy among the phases of matter ...
The Law of Definite Proportions
... • What pressure in atm will 1.36 kg of N2O gas exert when it is compressed in a 25.0 L cylinder and is stored in an outdoor shed where the temperature can reach 59°C in summer? ...
... • What pressure in atm will 1.36 kg of N2O gas exert when it is compressed in a 25.0 L cylinder and is stored in an outdoor shed where the temperature can reach 59°C in summer? ...
Chem 150 - Fall 2015 Exam I
... Element symbols and names: symbols, names, and spellings are recommended by IUPAC (http://www.iupac.org/). Names are not yet proposed for the elements beyond 111 - those used here are IUPAC’s temporary systematic names (Pure & Appl. Chem., 1979, 51, 381–384). In the USA and some other countries, the ...
... Element symbols and names: symbols, names, and spellings are recommended by IUPAC (http://www.iupac.org/). Names are not yet proposed for the elements beyond 111 - those used here are IUPAC’s temporary systematic names (Pure & Appl. Chem., 1979, 51, 381–384). In the USA and some other countries, the ...
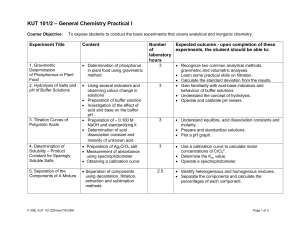
KUT 101/2 – General Chemistry Practical I
... dissociation constant and molarity of unknown acid • Preparation of Ag2CrO4 salt • Measurement of absorbance using spectrophotometer • Obtaining a calibration curve ...
... dissociation constant and molarity of unknown acid • Preparation of Ag2CrO4 salt • Measurement of absorbance using spectrophotometer • Obtaining a calibration curve ...
Student Worksheet The Chemistry of Water Quality Tests
... an example, colloids may be heterogeneous on the scale of micrometers, but homogeneous on the scale of centimeters. b. Solutions come in the form of solids, liquids, and gases. c. For liquid solutions, the solute may be a gas, a liquid, or a solid. Page 4 of 8 Developed by John Hnatow, AP Chemistry ...
... an example, colloids may be heterogeneous on the scale of micrometers, but homogeneous on the scale of centimeters. b. Solutions come in the form of solids, liquids, and gases. c. For liquid solutions, the solute may be a gas, a liquid, or a solid. Page 4 of 8 Developed by John Hnatow, AP Chemistry ...
Atomic Structure
... • The electrons are arranged in energy levels within the electron cloud. • Each energy level or shell is labeled with a number or letter. For example K-Shell or energy level 1 • Each energy level or shell can hold a maximum number of electrons: #e- = 2n2 where n = energy level. Level 1 holds 2 elect ...
... • The electrons are arranged in energy levels within the electron cloud. • Each energy level or shell is labeled with a number or letter. For example K-Shell or energy level 1 • Each energy level or shell can hold a maximum number of electrons: #e- = 2n2 where n = energy level. Level 1 holds 2 elect ...
Chemical Reactions.
... Atomic symbols describe the type of atoms in the compound (copper→ Cu, sulfur→ S, oxygen→ O) subscript numbers appear after the atomic symbol and describe the number of atoms in the compound (1 copper, 1 sulfur, 4 oxygen) subscript letters describe the physical state of the compound: s = solid, l = ...
... Atomic symbols describe the type of atoms in the compound (copper→ Cu, sulfur→ S, oxygen→ O) subscript numbers appear after the atomic symbol and describe the number of atoms in the compound (1 copper, 1 sulfur, 4 oxygen) subscript letters describe the physical state of the compound: s = solid, l = ...
Final Exam Review Answers
... • A box with a volume of 22.4 L contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0C. Which of the following statements is true? • a. The total pressure in the box is 202.6 kPa. • b. The partial pressure of N2 and H2 are equal. • c. The total pressure is 101.3 kPa. • d. The partial pressure of ...
... • A box with a volume of 22.4 L contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0C. Which of the following statements is true? • a. The total pressure in the box is 202.6 kPa. • b. The partial pressure of N2 and H2 are equal. • c. The total pressure is 101.3 kPa. • d. The partial pressure of ...
BS5-Ch 2.
... the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers. • cements the idea that atoms react as complete (whole) particles. • chemical formulas indicate whole numbers of atoms- not fractions ...
... the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers. • cements the idea that atoms react as complete (whole) particles. • chemical formulas indicate whole numbers of atoms- not fractions ...
Itty-Bitty Atoms
... science? Would you like to be a scientist? If so, what would you like to study? What do you think scientists of the future will study? 4. Answer the following questions: a. Who is Dmitry Mendeleyev and what did he do? b. What is chemistry? c. How big are atoms? 5. When a teacher calls out a symbol f ...
... science? Would you like to be a scientist? If so, what would you like to study? What do you think scientists of the future will study? 4. Answer the following questions: a. Who is Dmitry Mendeleyev and what did he do? b. What is chemistry? c. How big are atoms? 5. When a teacher calls out a symbol f ...
Chemistry Subject Matter Requirements Part I: Content Domains for
... e. Predict periodic trends, including electronegativity, ionization energy, reactivity, and the relative sizes of ions and atoms. (Next Generation Science Standards for California Public Schools, Kindergarten through Grade Twelve, Grades Nine through Twelve, Physical Sciences: PS1.A) Understand the ...
... e. Predict periodic trends, including electronegativity, ionization energy, reactivity, and the relative sizes of ions and atoms. (Next Generation Science Standards for California Public Schools, Kindergarten through Grade Twelve, Grades Nine through Twelve, Physical Sciences: PS1.A) Understand the ...
Chapter 13 Notes
... In this reaction, carbon is losing electrons so it is undergoing oxidation. Iron is gaining back electrons it had lost to become a free element so it is undergoing reduction. This is called an oxidation-reduction reaction or redox for short. Although this type of reaction is named for oxygen, many o ...
... In this reaction, carbon is losing electrons so it is undergoing oxidation. Iron is gaining back electrons it had lost to become a free element so it is undergoing reduction. This is called an oxidation-reduction reaction or redox for short. Although this type of reaction is named for oxygen, many o ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.