Midterm Review 2017
... 74) Which device should be used to accurately measure a volume of 16.30 milliliters? ...
... 74) Which device should be used to accurately measure a volume of 16.30 milliliters? ...
Chapter 2 BIO 100 Chemistry
... • Covalent bond between two atoms of the same element is always nonpolar. •A covalent bond between atoms that have similar electronegativities is also nonpolar. •Because carbon and hydrogen do not differ greatly in electronegativities, the bonds of CH4 are nonpolar. ...
... • Covalent bond between two atoms of the same element is always nonpolar. •A covalent bond between atoms that have similar electronegativities is also nonpolar. •Because carbon and hydrogen do not differ greatly in electronegativities, the bonds of CH4 are nonpolar. ...
FYBSc Revised Syllabus
... acid on primary, secondary and tertiary amines, Methylation of primary, secondary and tertiary amines, yielding quaternary ammonium salts; Hoffmann elimination. Note: Each reaction should be studied with respect to compounds with up to six carbon atoms. Based on these and the reactions of alkanes, a ...
... acid on primary, secondary and tertiary amines, Methylation of primary, secondary and tertiary amines, yielding quaternary ammonium salts; Hoffmann elimination. Note: Each reaction should be studied with respect to compounds with up to six carbon atoms. Based on these and the reactions of alkanes, a ...
Definitions - Loreto Science
... • is a substance which can be obtained in a pure stable soluble solid form so that it can be weighed out and dissolved in water to give a solution of accurately known ...
... • is a substance which can be obtained in a pure stable soluble solid form so that it can be weighed out and dissolved in water to give a solution of accurately known ...
File first semester final study guide key
... The periodic table organizes elements by putting them in __groups__________ or the elements in a vertical column on the periodic table and by _periods____ which are the elements in a horizontal row on the periodic table. Elements are placed in the periodic table in numerical order according to their ...
... The periodic table organizes elements by putting them in __groups__________ or the elements in a vertical column on the periodic table and by _periods____ which are the elements in a horizontal row on the periodic table. Elements are placed in the periodic table in numerical order according to their ...
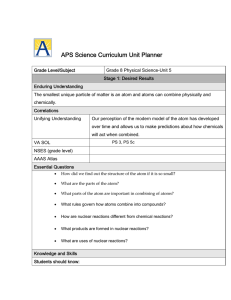
APS Science Curriculum Unit Planner
... The smallest unique particle of matter is an atom and atoms can combine physically and chemically. Correlations Unifying Understanding ...
... The smallest unique particle of matter is an atom and atoms can combine physically and chemically. Correlations Unifying Understanding ...
How Good Is the Quantum Mechanical Explanation of the Periodic
... The use of quantum mechanics, or more specifically, orbitals and electronic configurations in teaching general chemistry is now such a widespread trend that it would be utterly futile to try to reverse it. Moreover, orbitals and configurations have been extremely useful in providing a theoretical fr ...
... The use of quantum mechanics, or more specifically, orbitals and electronic configurations in teaching general chemistry is now such a widespread trend that it would be utterly futile to try to reverse it. Moreover, orbitals and configurations have been extremely useful in providing a theoretical fr ...
All you need to know about Additional Science
... different isotopes of chlorine. This is calculated by working out the relative abundance of each isotope. For example, in any sample of Chlorine 25% will be 37 Cl and 75% 35 Cl. The relative atomic mass is therefore calculated using the equation: • (% of isotope 1 × mass of isotope 1) + (% of isotop ...
... different isotopes of chlorine. This is calculated by working out the relative abundance of each isotope. For example, in any sample of Chlorine 25% will be 37 Cl and 75% 35 Cl. The relative atomic mass is therefore calculated using the equation: • (% of isotope 1 × mass of isotope 1) + (% of isotop ...
chapter 2 - Scranton Prep Biology
... in the object's position. Weight is the measureof how strongly an object is pulled by earth's gravity, and it varies with distance from the earth's center. Thi key point is that the mass of a body does not vary with its position, whereasweight does. So, for all practical purposes-as long as we are e ...
... in the object's position. Weight is the measureof how strongly an object is pulled by earth's gravity, and it varies with distance from the earth's center. Thi key point is that the mass of a body does not vary with its position, whereasweight does. So, for all practical purposes-as long as we are e ...
Notes - Ch 2
... 1. qualitative…what does it contain, and 2. quantitative…how much of everything does it contain B) Stoichiometry – composition stoichiometry (this chapter) and reaction stoichiometry (ch 3) 2-1 Atoms and Molecules A) Aristotle v Democritus 1. Early scientists – philosophers/thinkers – NOT experiment ...
... 1. qualitative…what does it contain, and 2. quantitative…how much of everything does it contain B) Stoichiometry – composition stoichiometry (this chapter) and reaction stoichiometry (ch 3) 2-1 Atoms and Molecules A) Aristotle v Democritus 1. Early scientists – philosophers/thinkers – NOT experiment ...
AP Chem Summer Assignment
... Writing chemical equations is also an essential skill. In Chem H, you used the Reference Tables help you predict products of reactions. In AP Chem, you have to memorize all of that information and more! Don’t worry; you’ll learn it a little at a time. But, you should be able to do it with the Table ...
... Writing chemical equations is also an essential skill. In Chem H, you used the Reference Tables help you predict products of reactions. In AP Chem, you have to memorize all of that information and more! Don’t worry; you’ll learn it a little at a time. But, you should be able to do it with the Table ...
New substances are formed by chemical reactions. When elements
... Covalent bonds Compounds formed from non-metals consist of molecules. The atoms in a molecule are joined together by covalent bonds. These bonds form when atoms share pairs of electrons. Chemical formulas The chemical formula of a compound shows how many of each type of atom join together to make th ...
... Covalent bonds Compounds formed from non-metals consist of molecules. The atoms in a molecule are joined together by covalent bonds. These bonds form when atoms share pairs of electrons. Chemical formulas The chemical formula of a compound shows how many of each type of atom join together to make th ...
Chemical Reactions
... Temperature Change (endo/exo-thermic) Formation of a Gas Formation of a Solid (Precipitate) ...
... Temperature Change (endo/exo-thermic) Formation of a Gas Formation of a Solid (Precipitate) ...
H 2 and H 2 + O 2 g H 2 O and H 2 O Hydrogen + Oxygen g Water
... Complete the following practical, ensuring you identify the mass of reactants and products (remember, we want to see if there was any change in mass)! Measure out 50ml of hydrochloric acid (HCl) into a beaker (find its mass) Measure out 50ml of copper sulfate (CuSO4) into a beaker (find its ma ...
... Complete the following practical, ensuring you identify the mass of reactants and products (remember, we want to see if there was any change in mass)! Measure out 50ml of hydrochloric acid (HCl) into a beaker (find its mass) Measure out 50ml of copper sulfate (CuSO4) into a beaker (find its ma ...
IPC Semester Exam Review – Chemistry Topics
... After reading cooking instructions that said to add salt to water before boiling it, Jose guessed that adding salt must make the water boil at a higher temperature. He decided to test his idea by performing the following experiment. Jose measured out 1 quart of distilled water and added to it 2 tabl ...
... After reading cooking instructions that said to add salt to water before boiling it, Jose guessed that adding salt must make the water boil at a higher temperature. He decided to test his idea by performing the following experiment. Jose measured out 1 quart of distilled water and added to it 2 tabl ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.