Chapter 2 - OrgSites.com
... 14. Describe the relationship between an electron’s potential energy and the distance it is from the nucleus. 15. When an electron absorbs energy, they are called “excited.” When an electron loses energy, it “falls back.” Give an example of each of these processes. ...
... 14. Describe the relationship between an electron’s potential energy and the distance it is from the nucleus. 15. When an electron absorbs energy, they are called “excited.” When an electron loses energy, it “falls back.” Give an example of each of these processes. ...
Chemistry! - Duplin County Schools
... • Words you need to know: – Magnetism – a force between like or unlike poles – Solubility – ability to dissolve – Density – mass per unit volume (mass divided by volume) ...
... • Words you need to know: – Magnetism – a force between like or unlike poles – Solubility – ability to dissolve – Density – mass per unit volume (mass divided by volume) ...
1-Three states of matter . A: density, volume and weight B: solid
... b. a noble gas in period 6 c. a main group element in period 3 that has p orbitals half-fi lled with electrons d. a transition metal in period 4, group 11 e. an inner transition metal with its 5f orbitals completely f. a transition metal in period 6, group 10 filled with electrons 7- Label each regi ...
... b. a noble gas in period 6 c. a main group element in period 3 that has p orbitals half-fi lled with electrons d. a transition metal in period 4, group 11 e. an inner transition metal with its 5f orbitals completely f. a transition metal in period 6, group 10 filled with electrons 7- Label each regi ...
Chemistry Note PowerPoint
... • The space is huge compared to the amount of space taken by the nucleus. • It symbolizes where electrons are LIKELY to be. • An electrons movement is related to its energy level, or the specific amount of energy that it has. ...
... • The space is huge compared to the amount of space taken by the nucleus. • It symbolizes where electrons are LIKELY to be. • An electrons movement is related to its energy level, or the specific amount of energy that it has. ...
Document
... It is important that atoms bond. Why? Because they need to bond in order to make _____________, _______________, and other more complex forms of matter. For example, if atoms didn’t bond, you would be quite thirsty all the time! Yes, ______________ is the result of the process of CHEMICAL bonding. T ...
... It is important that atoms bond. Why? Because they need to bond in order to make _____________, _______________, and other more complex forms of matter. For example, if atoms didn’t bond, you would be quite thirsty all the time! Yes, ______________ is the result of the process of CHEMICAL bonding. T ...
4.1Atoms and Isotopes
... Carbon has three isotopes: C-12 (most abundant), C-13 (used in medical imagingMRI), and C-14 (used for dating fossils) Tin (Sn) has the most isotopes of any element at 10 Many isotopes are radioactive (unstable nucleus that will eventually break apart and release energy in sometimes harmful forms – ...
... Carbon has three isotopes: C-12 (most abundant), C-13 (used in medical imagingMRI), and C-14 (used for dating fossils) Tin (Sn) has the most isotopes of any element at 10 Many isotopes are radioactive (unstable nucleus that will eventually break apart and release energy in sometimes harmful forms – ...
atomic number
... nucleus of an atom is called the atomic number. For example, any atom with 6 protons in the nucleus is a Carbon atom. • Elements are arranged in the periodic table by their atomic number. • In a neutral atom, # electrons = #protons. • The symbol for an element is simply its 1, 2, or 3 letter abbrevi ...
... nucleus of an atom is called the atomic number. For example, any atom with 6 protons in the nucleus is a Carbon atom. • Elements are arranged in the periodic table by their atomic number. • In a neutral atom, # electrons = #protons. • The symbol for an element is simply its 1, 2, or 3 letter abbrevi ...
Bio 102 Lecture - chapter 2 The Chemical Basis of Life
... If 3 or less electrons in the outer most shell – Tendency to donate electrons. If 5 or more electrons in the outer most shell – Tendency to receive electrons. A ‘chemical bond’ the force of attraction between atoms to attain stability. ...
... If 3 or less electrons in the outer most shell – Tendency to donate electrons. If 5 or more electrons in the outer most shell – Tendency to receive electrons. A ‘chemical bond’ the force of attraction between atoms to attain stability. ...
Chapter 2 - Phillips Scientific Methods
... • This can be abbreviated further with a molecular formula. – For example, H2 ...
... • This can be abbreviated further with a molecular formula. – For example, H2 ...
Honors Chemistry Exam Review Questions
... B The scientific method is a logical, systematic approach to the solution of a problem. C For the results of an experiment to be accepted, the experiment must produce the same results no matter how many times it is repeated. D The scientific process is repeated until a hypothesis either fits all the ...
... B The scientific method is a logical, systematic approach to the solution of a problem. C For the results of an experiment to be accepted, the experiment must produce the same results no matter how many times it is repeated. D The scientific process is repeated until a hypothesis either fits all the ...
(1) Dissolves, accompanied by evolution of flammable gas (2
... The phase diagram for a pure substance is shown above. Use this diagram and your knowledge about changes of phase to answer the following questions. (a) What does point V represent? What characteristics are specific to the system only at point V ? (b) What does each point on the curve between V and ...
... The phase diagram for a pure substance is shown above. Use this diagram and your knowledge about changes of phase to answer the following questions. (a) What does point V represent? What characteristics are specific to the system only at point V ? (b) What does each point on the curve between V and ...
compound - Coal City Unit #1
... • a class of compounds whose water solns. taste sour, turn blue litmus paper red, and react with bases to form salts (ionic compounds) ...
... • a class of compounds whose water solns. taste sour, turn blue litmus paper red, and react with bases to form salts (ionic compounds) ...
O - gearju.com
... (a) The electronegativity difference between H and Cl is 0.9, which is appreciable but not large enough (by the 2.0 rule) to qualify HCl as an ionic compound. Therefore, the bond between H and Cl is polar covalent. (b) The electronegativity difference between K and F is 3.2, which is well above the ...
... (a) The electronegativity difference between H and Cl is 0.9, which is appreciable but not large enough (by the 2.0 rule) to qualify HCl as an ionic compound. Therefore, the bond between H and Cl is polar covalent. (b) The electronegativity difference between K and F is 3.2, which is well above the ...
CHEMISTRY IM 06 SYLLABUS
... Section B will consist of five compulsory structured questions; Section C will require candidates to choose two out of four long questions. Each section carries equal marks. The minimum mathematical requirements of the syllabus are the same as those for the SEC examination in Chemistry. Questions wi ...
... Section B will consist of five compulsory structured questions; Section C will require candidates to choose two out of four long questions. Each section carries equal marks. The minimum mathematical requirements of the syllabus are the same as those for the SEC examination in Chemistry. Questions wi ...
O - gearju.com
... (a) The electronegativity difference between H and Cl is 0.9, which is appreciable but not large enough (by the 2.0 rule) to qualify HCl as an ionic compound. Therefore, the bond between H and Cl is polar covalent. (b) The electronegativity difference between K and F is 3.2, which is well above the ...
... (a) The electronegativity difference between H and Cl is 0.9, which is appreciable but not large enough (by the 2.0 rule) to qualify HCl as an ionic compound. Therefore, the bond between H and Cl is polar covalent. (b) The electronegativity difference between K and F is 3.2, which is well above the ...
CHEMISTRY IM 06 SYLLABUS
... Section B will consist of five compulsory structured questions; Section C will require candidates to choose two out of four long questions. Each section carries equal marks. The minimum mathematical requirements of the syllabus are the same as those for the SEC examination in Chemistry. Questions wi ...
... Section B will consist of five compulsory structured questions; Section C will require candidates to choose two out of four long questions. Each section carries equal marks. The minimum mathematical requirements of the syllabus are the same as those for the SEC examination in Chemistry. Questions wi ...
Document
... Isotopes, Atomic Numbers, and Mass Numbers •Atomic number (Z) = number of protons in the nucleus. •Mass number (A) = total number of nucleons in the nucleus (i.e., protons and neutrons). ...
... Isotopes, Atomic Numbers, and Mass Numbers •Atomic number (Z) = number of protons in the nucleus. •Mass number (A) = total number of nucleons in the nucleus (i.e., protons and neutrons). ...
CHEMISTRY 102B Name Hour Exam II March 19, 2015 Signature
... b) The ground state electron configuration for the most stable ion of sodium in a compound is 1s22s22p 6. c) The ground state electron configuration for the valence electrons of the halogens (Group 7A) is ns2np5. d) At least two of the above statements (a-c) are false. e) All of the above statements ...
... b) The ground state electron configuration for the most stable ion of sodium in a compound is 1s22s22p 6. c) The ground state electron configuration for the valence electrons of the halogens (Group 7A) is ns2np5. d) At least two of the above statements (a-c) are false. e) All of the above statements ...
Total Notes for chem - Catawba County Schools
... based on these ideas; 1. When elements combine, the outermost electrons; that is, those electrons with levels of energy which will cause them to orbit at the greatest distance from the nucleus, will be the only electrons directly involved with the reaction of these elements to form compounds. 2. The ...
... based on these ideas; 1. When elements combine, the outermost electrons; that is, those electrons with levels of energy which will cause them to orbit at the greatest distance from the nucleus, will be the only electrons directly involved with the reaction of these elements to form compounds. 2. The ...
Quarterly 1 Review Trupia - Trupia
... ____53. Which element has a total of 5 valence electrons present in the fifth energy level (shell)? ____60. Which element forms an ion that is larger (1) Sb (3) I than its atom? (2) Bi (4) Br (1) aluminum (3) magnesium (2) chlorine (4) sodium ____54. Lithium and potassium have similar chemical prope ...
... ____53. Which element has a total of 5 valence electrons present in the fifth energy level (shell)? ____60. Which element forms an ion that is larger (1) Sb (3) I than its atom? (2) Bi (4) Br (1) aluminum (3) magnesium (2) chlorine (4) sodium ____54. Lithium and potassium have similar chemical prope ...
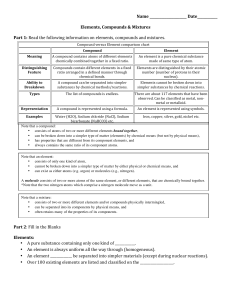
Compound vs Element chart
... • consists of only one kind of atom, • cannot be broken down into a simpler type of matter by either physical or chemical means, and • can exist as either atoms (e.g. argon) or molecules (e.g., nitrogen). A molecule consists of two or more atoms of the same element, or different elements, that are c ...
... • consists of only one kind of atom, • cannot be broken down into a simpler type of matter by either physical or chemical means, and • can exist as either atoms (e.g. argon) or molecules (e.g., nitrogen). A molecule consists of two or more atoms of the same element, or different elements, that are c ...
C. Adding acid shifts the equilibrium to the right
... When the atoms in a bond are the same, the electrons are shared equally. This results in a nonpolar covalent bond. Diatomic elements (Br2, I2 N2, Cl2, H2, O2 and F2) have pure nonpolar covalent bonds. Nonpolar molecules are symmetrical because there are no unshared electrons around the central atom. ...
... When the atoms in a bond are the same, the electrons are shared equally. This results in a nonpolar covalent bond. Diatomic elements (Br2, I2 N2, Cl2, H2, O2 and F2) have pure nonpolar covalent bonds. Nonpolar molecules are symmetrical because there are no unshared electrons around the central atom. ...
Page | 1 MATS1101 Chemistry notes semester 2 2012 TOPIC 1
... Using this theory we can explain three fundamental laws of chemical behaviour: 1. Law of Conservation of Mass and Energy: Matter is neither created or destroyed in a chemical reaction. Energy is neither created or destroyed in a chemical reaction, but it may be transformed from one form to another. ...
... Using this theory we can explain three fundamental laws of chemical behaviour: 1. Law of Conservation of Mass and Energy: Matter is neither created or destroyed in a chemical reaction. Energy is neither created or destroyed in a chemical reaction, but it may be transformed from one form to another. ...
Electronegativity

Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom or a functional group to attract electrons (or electron density) towards itself. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity number, the more an element or compound attracts electrons towards it. The term ""electronegativity"" was introduced by Jöns Jacob Berzelius in 1811,though the concept was known even before that and was studied by many chemists including Avogadro.In spite of its long history, an accurate scale of electronegativity had to wait till 1932, when Linus Pauling proposed an electronegativity scale, which depends on bond energies, as a development of valence bond theory. It has been shown to correlate with a number of other chemical properties. Electronegativity cannot be directly measured and must be calculated from other atomic or molecular properties. Several methods of calculation have been proposed, and although there may be small differences in the numerical values of the electronegativity, all methods show the same periodic trends between elements. The most commonly used method of calculation is that originally proposed by Linus Pauling. This gives a dimensionless quantity, commonly referred to as the Pauling scale, on a relative scale running from around 0.7 to 3.98 (hydrogen = 2.20). When other methods of calculation are used, it is conventional (although not obligatory) to quote the results on a scale that covers the same range of numerical values: this is known as an electronegativity in Pauling units. As it is usually calculated, electronegativity is not a property of an atom alone, but rather a property of an atom in a molecule. Properties of a free atom include ionization energy and electron affinity. It is to be expected that the electronegativity of an element will vary with its chemical environment, but it is usually considered to be a transferable property, that is to say that similar values will be valid in a variety of situations.On the most basic level, electronegativity is determined by factors like the nuclear charge (the more protons an atom has, the more ""pull"" it will have on electrons) and the number/location of other electrons present in the atomic shells (the more electrons an atom has, the farther from the nucleus the valence electrons will be, and as a result the less positive charge they will experience—both because of their increased distance from the nucleus, and because the other electrons in the lower energy core orbitals will act to shield the valence electrons from the positively charged nucleus).The opposite of electronegativity is electropositivity: a measure of an element's ability to donate electrons.Caesium is the least electronegative element in the periodic table (=0.79), while fluorine is most electronegative (=3.98). (Francium and caesium were originally assigned both assigned 0.7; caesium's value was later refined to 0.79, but no experimental data allows a similar refinement for francium. However, francium's ionization energy is known to be slightly higher than caesium's, in accordance with the relativistic stabilization of the 7s orbital, and this in turn implies that caesium is in fact more electronegative than francium.)