
NAME PRACTICE: QUANTUM CONFIGURATIONS 1) Each of the
... ___20) The ground-state configuration for the atoms of a transition element ___21) The ground-state configuration of a negative ion of a halogen ___22) The ground-state configuration of a common ion of an alkaline earth element Use these answers for questions 23-25. (1) Heisenberg uncertainty princi ...
... ___20) The ground-state configuration for the atoms of a transition element ___21) The ground-state configuration of a negative ion of a halogen ___22) The ground-state configuration of a common ion of an alkaline earth element Use these answers for questions 23-25. (1) Heisenberg uncertainty princi ...
chapter 7: atomic structure and periodicity
... A New Model – Quantum (Wave) Mechanical Model. A mathematical model developed by __________________________, ____________________________, and ___________________ treats the electron as if it were a _____________________. ______________________ originated the idea that small particles, such as the e ...
... A New Model – Quantum (Wave) Mechanical Model. A mathematical model developed by __________________________, ____________________________, and ___________________ treats the electron as if it were a _____________________. ______________________ originated the idea that small particles, such as the e ...
Bohr Model, Quantum Mechanical Model
... b. energy is involved in moving an electron from one level to another. 4. Heisenberg Uncertainty Principle- It is impossible to know the momentum (mass of electron times velocity) of an electron and its position in space at the same time. One or the other. 5. Quantum Mechanical Model- a mathematical ...
... b. energy is involved in moving an electron from one level to another. 4. Heisenberg Uncertainty Principle- It is impossible to know the momentum (mass of electron times velocity) of an electron and its position in space at the same time. One or the other. 5. Quantum Mechanical Model- a mathematical ...
Nuclear Fission sim
... Why do fission and fusion produce so much energy? • In nuclear reactions, energy comes from converting tiny amounts of mass lost when the bonds between protons and neutrons are broken and made. These bonds are due to the strong force. • The strong force is a thousand times stronger than the electri ...
... Why do fission and fusion produce so much energy? • In nuclear reactions, energy comes from converting tiny amounts of mass lost when the bonds between protons and neutrons are broken and made. These bonds are due to the strong force. • The strong force is a thousand times stronger than the electri ...
Bohr vs. Correct Model of Atom
... • h (Planck’s constant) = 6.63 x 10-34 J-s • 1 eV = kinetic energy of an electron that has been accelerated through a potential difference of 1 V 1 eV = q x V = 1.6 x 10-19 J ...
... • h (Planck’s constant) = 6.63 x 10-34 J-s • 1 eV = kinetic energy of an electron that has been accelerated through a potential difference of 1 V 1 eV = q x V = 1.6 x 10-19 J ...
ionization energies
... • In order to go from Be to Be3+, you must LOSE 3 electrons. This will require 3 ionization steps (see pg 107 in book). ...
... • In order to go from Be to Be3+, you must LOSE 3 electrons. This will require 3 ionization steps (see pg 107 in book). ...
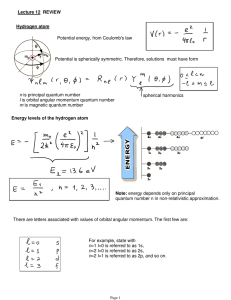
Lecture 12: Review.
... Example: hydrogen ground state J=1/2, I=1/2 (proton) F=0,1, so two levels (see picture on previous page) ...
... Example: hydrogen ground state J=1/2, I=1/2 (proton) F=0,1, so two levels (see picture on previous page) ...
Chapter 2 Chemistry comes alive
... Valence shell – outermost energy level containing chemically active electrons Octet rule – except for the first shell which is full with two electrons, atoms interact in a manner to have eight electrons in their valence shell ...
... Valence shell – outermost energy level containing chemically active electrons Octet rule – except for the first shell which is full with two electrons, atoms interact in a manner to have eight electrons in their valence shell ...
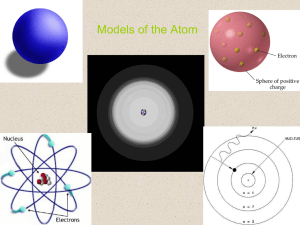
Models of the Atom
... The Bohr Model • Niels Bohr (1885 – 1962) – Danish Physicist/ Student of Rutherford – An electron is found only in specific circular paths, or orbits, around the nucleus. – The energy of an atom changes when it absorbs or emits light. – Each orbit has a fixed energy (each orbital is a different ene ...
... The Bohr Model • Niels Bohr (1885 – 1962) – Danish Physicist/ Student of Rutherford – An electron is found only in specific circular paths, or orbits, around the nucleus. – The energy of an atom changes when it absorbs or emits light. – Each orbit has a fixed energy (each orbital is a different ene ...
Atomic Structure and Electron Configurations Multiple Choice PSI
... Atomic Structure and Electron Configurations Multiple Choice PSI Chemistry Name:________________________ 1. Rutherford’s Nuclear Model of the atom A. is the currently accepted atomic model. B. explains the unique emission spectra of different elements. C. does not account for the stability of most a ...
... Atomic Structure and Electron Configurations Multiple Choice PSI Chemistry Name:________________________ 1. Rutherford’s Nuclear Model of the atom A. is the currently accepted atomic model. B. explains the unique emission spectra of different elements. C. does not account for the stability of most a ...
Unit 3 – Quantum Mechanical Model of the Atom
... energy, it jumps from its ground state to an excited state. • When the electron falls back to the ground state, energy is given off in the form of light. • Bohr used Planck’s equation, E = hv, to verify this theory for hydrogen. ...
... energy, it jumps from its ground state to an excited state. • When the electron falls back to the ground state, energy is given off in the form of light. • Bohr used Planck’s equation, E = hv, to verify this theory for hydrogen. ...
Revision topic 1-3
... In every atom there is a balance between the attraction of the positively charged nucleus for the negatively charged electrons and repulsion between the electrons. The outer electrons (valence electrons) do not experience the full attraction of the positive nucleus because of the presence of inner e ...
... In every atom there is a balance between the attraction of the positively charged nucleus for the negatively charged electrons and repulsion between the electrons. The outer electrons (valence electrons) do not experience the full attraction of the positive nucleus because of the presence of inner e ...
The Modern Atomic Model
... • Each orbital, in each energy level can only hold a maximum of 2 e-. • s sublevel 1 orbital, 2 e• p sublevel 3 orbitals, 6 e• d sublevel 5 orbitals, 10 e• f sublevel 7 orbitals, 14 e- ...
... • Each orbital, in each energy level can only hold a maximum of 2 e-. • s sublevel 1 orbital, 2 e• p sublevel 3 orbitals, 6 e• d sublevel 5 orbitals, 10 e• f sublevel 7 orbitals, 14 e- ...
Electronic Structure of Atoms (i.e., Quantum Mechanics)
... Carbon: Contains many more emission lines as compared to H. Why? ...
... Carbon: Contains many more emission lines as compared to H. Why? ...
















![[30 pts] While the spins of the two electrons in a hydrog](http://s1.studyres.com/store/data/002487557_1-ac2bceae20801496c3356a8afebed991-300x300.png)




