![L 35 Modern Physics [1]](http://s1.studyres.com/store/data/008517000_1-9aef89c0ca089782f518550164188024-300x300.png)
In-Class Exam - Fayetteville State University
... 14. Isotopes are atoms that have the same number of ______ but differing number of ______. A) neutrons, protons B) protons, electrons C) neutrons, electrons D) electrons, protons ...
... 14. Isotopes are atoms that have the same number of ______ but differing number of ______. A) neutrons, protons B) protons, electrons C) neutrons, electrons D) electrons, protons ...
N/Z = 2, 8, 20, 28, 50, 82, 126
... • empirically, the sign of the spin-orbit term for nuclei is opposite to that for atoms and the effect is much stronger in nuclei – the phenomenon has nothing to do with magnetism, which is the origin of this effect in atoms, but rather it reflects a basic feature of the strong nuclear force. • with ...
... • empirically, the sign of the spin-orbit term for nuclei is opposite to that for atoms and the effect is much stronger in nuclei – the phenomenon has nothing to do with magnetism, which is the origin of this effect in atoms, but rather it reflects a basic feature of the strong nuclear force. • with ...
Lecture
... Spin quantum number “s” is a unique property of a particle. Fermions have half integer value of “s”. Two fermions cannot occupy the same quantum state. Electron, Proton, Neutron: s=1/2 Bosons have full integer value of “s”. There is no limitation in the number of bosons that can occupy the same stat ...
... Spin quantum number “s” is a unique property of a particle. Fermions have half integer value of “s”. Two fermions cannot occupy the same quantum state. Electron, Proton, Neutron: s=1/2 Bosons have full integer value of “s”. There is no limitation in the number of bosons that can occupy the same stat ...
CM1111* Question 1 (40 marks) Multiple Choice Questions, 5 marks
... B. Nitrogen has a lower electron affinity than oxygen C. The 2p orbital has no radial nodes but has an angular node D. For an s orbital, at a particular distance from the nucleus, the probability of finding the electron is equal in all directions. (5) Which of the following statement(s) is/are not t ...
... B. Nitrogen has a lower electron affinity than oxygen C. The 2p orbital has no radial nodes but has an angular node D. For an s orbital, at a particular distance from the nucleus, the probability of finding the electron is equal in all directions. (5) Which of the following statement(s) is/are not t ...
Q: In which model of the atom do electrons orbit the nucleus? A
... Q: In which model of the atom were electrons discovered (corpuscles) and reside along with the protons like raisins in plum pudding? (using cathode rays) A: Thomson ...
... Q: In which model of the atom were electrons discovered (corpuscles) and reside along with the protons like raisins in plum pudding? (using cathode rays) A: Thomson ...
AP Chemistry 2013 Semester 1 Final Exam Review Problems
... mass (in amu) of a carbon sample prepared by mixing equal numbers of carbon atoms from a sample of natural carbon and a sample of pure carbon-13? 4. A monoatomic ion has a charge of 2+. The nucleus of the ion has a mass number of 62. The number of neutrons in the nucleus is 1.21 times that of the nu ...
... mass (in amu) of a carbon sample prepared by mixing equal numbers of carbon atoms from a sample of natural carbon and a sample of pure carbon-13? 4. A monoatomic ion has a charge of 2+. The nucleus of the ion has a mass number of 62. The number of neutrons in the nucleus is 1.21 times that of the nu ...
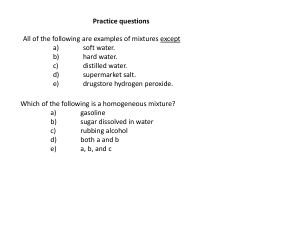
Practice questions
... The average atomic mass of Eu is 151.96 amu. There are only two naturally occurring isotopes of europium, 151Eu with a mass of 151.0 amu and 153Eu with a mass of 153.0 amu. The natural abundance of the 131Eu isotope must be approximately a) ...
... The average atomic mass of Eu is 151.96 amu. There are only two naturally occurring isotopes of europium, 151Eu with a mass of 151.0 amu and 153Eu with a mass of 153.0 amu. The natural abundance of the 131Eu isotope must be approximately a) ...
REGAN-Emanuel-June2013-FINAL
... Internal structures, gamma rays and shells. How big is the nuclear chart ? What could this tell us about nucleosynthesis? ...
... Internal structures, gamma rays and shells. How big is the nuclear chart ? What could this tell us about nucleosynthesis? ...
Multi-electron atoms have interactions between electrons, not just
... When we talk about describing electrons ... we will talk about the PARAMETERS that go into this WAVEFUNCTION ... without doing the actual math. - There are FOUR of these parameters. (the Bohr model had only one!) - The parameters are called "quantum numbers" Principal quantum number Angular momentum ...
... When we talk about describing electrons ... we will talk about the PARAMETERS that go into this WAVEFUNCTION ... without doing the actual math. - There are FOUR of these parameters. (the Bohr model had only one!) - The parameters are called "quantum numbers" Principal quantum number Angular momentum ...
Historical Introduction to the Elementary Particles 2
... populated with protons, neutrons, and electrons, instead of antiprotons, antineutrons, and positrons? Matter and antimatter cannot coexist for long if a particle meets its antiparticle, they annihilate. So maybe it’s just a historical accident that in our comer of the universe there happened to be m ...
... populated with protons, neutrons, and electrons, instead of antiprotons, antineutrons, and positrons? Matter and antimatter cannot coexist for long if a particle meets its antiparticle, they annihilate. So maybe it’s just a historical accident that in our comer of the universe there happened to be m ...
Quantum Theory of the Atom
... D. Electrons do not move around the nucleus in circular orbits E. Laid ground for later atomic ...
... D. Electrons do not move around the nucleus in circular orbits E. Laid ground for later atomic ...