Physical Properties and Acidity of Carboxylic Acids
... ten times weaker an acid than formic acid (first two entries in the second row), confirming the electron donating character of an alkyl group relative to hydrogen, as noted earlier in a discussion of carbocation stability. Electronegative substituents increase acidity by inductive electron withdrawa ...
... ten times weaker an acid than formic acid (first two entries in the second row), confirming the electron donating character of an alkyl group relative to hydrogen, as noted earlier in a discussion of carbocation stability. Electronegative substituents increase acidity by inductive electron withdrawa ...
Microsoft Word
... cyclic ketone were dissolved in dichloromethane, and the resulting clear solution was stirred at room temperature for 8-12 hours allowed to formation of spiroisoxazoline in 71-89% yield. This encouraging result prompted us to study several other nitrile oxides and a-methylene cyclic ketones for sim ...
... cyclic ketone were dissolved in dichloromethane, and the resulting clear solution was stirred at room temperature for 8-12 hours allowed to formation of spiroisoxazoline in 71-89% yield. This encouraging result prompted us to study several other nitrile oxides and a-methylene cyclic ketones for sim ...
Unit 3: Bonding and Nomenclature Content Outline: Chemical
... A. The natural tendency is to achieve the lowest possible Potential Energy state and thus behave “like” a Noble gas element. B. Energy is released in bond formation between atoms. C. Energy is required in the breaking of a bond between atoms. 1. The energy to make or break a bond is referred to as b ...
... A. The natural tendency is to achieve the lowest possible Potential Energy state and thus behave “like” a Noble gas element. B. Energy is released in bond formation between atoms. C. Energy is required in the breaking of a bond between atoms. 1. The energy to make or break a bond is referred to as b ...
Electron Delocalization, Resonance and Aromaticity
... The resonance energy tells us how much more stable the compound with delocalized electrons is due to that delocalization The greater the number of relatively stable resonance contributors, the greater the resonance energy ...
... The resonance energy tells us how much more stable the compound with delocalized electrons is due to that delocalization The greater the number of relatively stable resonance contributors, the greater the resonance energy ...
Chem 226 — Problem Set #2
... hydrogens of 2,5-dimethylhexane with the chlorine to get one of the monochloro derivatives. Then, starting again with 2,5-dimethylhexane, we have to replace a different hydrogen with the chlorine in such a way that we get a different constitutional isomer from the one we already have. We continue in ...
... hydrogens of 2,5-dimethylhexane with the chlorine to get one of the monochloro derivatives. Then, starting again with 2,5-dimethylhexane, we have to replace a different hydrogen with the chlorine in such a way that we get a different constitutional isomer from the one we already have. We continue in ...
Activity - OChemOnline
... 13. (1 point) Circle the number that represents your best estimate of how many constitutional isomers with the molecular formula C2H5NO2 could one make. ...
... 13. (1 point) Circle the number that represents your best estimate of how many constitutional isomers with the molecular formula C2H5NO2 could one make. ...
Chapter 9 Chemical Bonding
... b) Draw the Lewis electron dot structure for each atom. (Use the method in which the electrons are spread to all four sides of an imaginary square before being paired.) For the sake of keeping the drawing as neat as possible, direct single electrons on adjacent atoms towards each other. c) Draw a li ...
... b) Draw the Lewis electron dot structure for each atom. (Use the method in which the electrons are spread to all four sides of an imaginary square before being paired.) For the sake of keeping the drawing as neat as possible, direct single electrons on adjacent atoms towards each other. c) Draw a li ...
Phenols Like alcohols, phenols are starting materials for a wide
... Like alcohols, phenols are starting materials for a wide range of compounds. The functional group is again OH but unlike alcohols, it is attached directly to a benzene ring and this affects its reactivity. ...
... Like alcohols, phenols are starting materials for a wide range of compounds. The functional group is again OH but unlike alcohols, it is attached directly to a benzene ring and this affects its reactivity. ...
Weiguang Zhang 1,* Yun Zhong 1, Minyu Tan 2,*, Ning Tang 2 and
... Investigation of the role that zinc thiolate complexes play in biological chemistry has stimulated numerous studies on zinc coordination compounds with sulfur ligation [1-4]. In particular, zinc complexes with NS2 ligand cores are of increasing interest as structural and spectroscopic models for met ...
... Investigation of the role that zinc thiolate complexes play in biological chemistry has stimulated numerous studies on zinc coordination compounds with sulfur ligation [1-4]. In particular, zinc complexes with NS2 ligand cores are of increasing interest as structural and spectroscopic models for met ...
zinc(II): Zn 2 [(n
... In general, coordination of a ligand to a metal ion causes an increase in the electron density on the metal, resulting in a decrease in the metal ion’s electron binding energy [12]. The Zn(2p)3/2 chemical shift, which are the differences between the Zn(2p)3/2 binding energies of the complex and ZnCl ...
... In general, coordination of a ligand to a metal ion causes an increase in the electron density on the metal, resulting in a decrease in the metal ion’s electron binding energy [12]. The Zn(2p)3/2 chemical shift, which are the differences between the Zn(2p)3/2 binding energies of the complex and ZnCl ...
CHAPTER 4 CARBON AND THE MOLECULAR DIVERSITY OF LIFE
... 1. Functional groups contribute to the molecular diversity of life • The components of organic molecules that are most commonly involved in chemical reactions are known as functional groups. • Functional groups are attachments that replace one or more hydrogen atoms to the carbon skeleton of the hy ...
... 1. Functional groups contribute to the molecular diversity of life • The components of organic molecules that are most commonly involved in chemical reactions are known as functional groups. • Functional groups are attachments that replace one or more hydrogen atoms to the carbon skeleton of the hy ...
Chemistry: The Central Science, 12e (Brown et al
... 39) Alkenes have the general formula __________. A) CnH2n. B) CnH2n-2. C) CnH2n+2 D) CnHn. E) C2nHn. 40) In general, __________ are the most reactive hydrocarbons. A) alkenes B) alkynes C) alkanes D) cycloalkanes E) olefins 41) The addition of HBr to 2-butene produces __________. A) 1-bromobutane B) ...
... 39) Alkenes have the general formula __________. A) CnH2n. B) CnH2n-2. C) CnH2n+2 D) CnHn. E) C2nHn. 40) In general, __________ are the most reactive hydrocarbons. A) alkenes B) alkynes C) alkanes D) cycloalkanes E) olefins 41) The addition of HBr to 2-butene produces __________. A) 1-bromobutane B) ...
SPECTRA Q - the British School of Bahrain
... A tablet of ibuprofen contains a very small quantity of the drug and the remainder of the tablet material is unreactive. In an analysis 50 tablets were reacted with 100.0 cm3 of 1.00 mol dm–3 aqueous sodium hydroxide, an excess. The ibuprofen reacted as a weak acid. When the reaction was complete, t ...
... A tablet of ibuprofen contains a very small quantity of the drug and the remainder of the tablet material is unreactive. In an analysis 50 tablets were reacted with 100.0 cm3 of 1.00 mol dm–3 aqueous sodium hydroxide, an excess. The ibuprofen reacted as a weak acid. When the reaction was complete, t ...
Organic Chemistry
... hydrocarbons. Structure, bonding, stereochemistry, conformational analysis nomenclature, and physical properties in relation to these particular groups of compounds. Emphasis on reactivity, and reaction mechanisms. Laboratory work includes microscale, macroscale, spectroscopic, and chromagraphic tec ...
... hydrocarbons. Structure, bonding, stereochemistry, conformational analysis nomenclature, and physical properties in relation to these particular groups of compounds. Emphasis on reactivity, and reaction mechanisms. Laboratory work includes microscale, macroscale, spectroscopic, and chromagraphic tec ...
Part One: Molecular Geometry and Directional Bonding A
... Descriptions of Multiple Bonding using VB Theory. ...
... Descriptions of Multiple Bonding using VB Theory. ...
Document
... equivalent) and ketone (0.2 mmol, 2 equivalent) in precooled toluene (20 °C, 0.5 mL), catalystQNI was added (6.0 mg, 0.01 mmol, 10 mol%) in one load. The reaction mixture was stirred for 1 min and placed in freezer (20 °C) for 2 days without stirring. The reaction mixture was monitored by 19F NMR fo ...
... equivalent) and ketone (0.2 mmol, 2 equivalent) in precooled toluene (20 °C, 0.5 mL), catalystQNI was added (6.0 mg, 0.01 mmol, 10 mol%) in one load. The reaction mixture was stirred for 1 min and placed in freezer (20 °C) for 2 days without stirring. The reaction mixture was monitored by 19F NMR fo ...
Chemistry 235, Winter 2008 Name: General rules:
... • In compounds, the more electronegative element (p. 137) is assigned the negative oxidation number. • Hydrogen in an organic compound is assigned an oxidation number of +1. • Oxygen in an organic compound generally is assigned an oxidation number of –2. • The sum of all of the oxidation numbers of ...
... • In compounds, the more electronegative element (p. 137) is assigned the negative oxidation number. • Hydrogen in an organic compound is assigned an oxidation number of +1. • Oxygen in an organic compound generally is assigned an oxidation number of –2. • The sum of all of the oxidation numbers of ...
Main Menu - MsReenChemistry
... Each person is a carbon atom. You are only allowed to link hands or one leg with one, two or three people. Assume any open hands or legs are bonded to an imaginary hydrogen atom. How many different arrangements can you make? ...
... Each person is a carbon atom. You are only allowed to link hands or one leg with one, two or three people. Assume any open hands or legs are bonded to an imaginary hydrogen atom. How many different arrangements can you make? ...
11. 5-member heterocycles with 1 and heteroatoms
... Imidazole was first synthesized by Heinrich Debus in 1858, but various imidazole derivatives had been discovered as early as the 1840s. His synthesis, as shown below, used glyoxal and formaldehyde in ammonia to form imidazole. This synthesis, while producing relatively low yields, is still used for ...
... Imidazole was first synthesized by Heinrich Debus in 1858, but various imidazole derivatives had been discovered as early as the 1840s. His synthesis, as shown below, used glyoxal and formaldehyde in ammonia to form imidazole. This synthesis, while producing relatively low yields, is still used for ...
III. Ionic Compounds
... Stock System - Use Roman numerals to show the ion’s charge if more than one is possible. Overall charge must equal zero. C. Johannesson ...
... Stock System - Use Roman numerals to show the ion’s charge if more than one is possible. Overall charge must equal zero. C. Johannesson ...
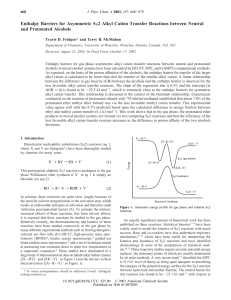
Enthalpy Barriers for Asymmetric SN2 Alkyl
... ion/molecule complex must attain a structure in which the oxygen of the neutral alcohol is bound to the R-carbon of the alkyl group of the protonated alcohol (Figure 2A).15 This “alkylbound” complex then undergoes an alkyl cation transfer, effectively from water to the neutral alcohol, producing the ...
... ion/molecule complex must attain a structure in which the oxygen of the neutral alcohol is bound to the R-carbon of the alkyl group of the protonated alcohol (Figure 2A).15 This “alkylbound” complex then undergoes an alkyl cation transfer, effectively from water to the neutral alcohol, producing the ...
Critical Thinking Question (cont`d.)
... What is Process Oriented Guided Inquiry Learning (POGIL)? POGIL is a classroom and laboratory technique that seeks to simultaneously teach content and key process skills such as the ability to think analytically and work effectively as part of a collaborative team. POGIL is based on research indica ...
... What is Process Oriented Guided Inquiry Learning (POGIL)? POGIL is a classroom and laboratory technique that seeks to simultaneously teach content and key process skills such as the ability to think analytically and work effectively as part of a collaborative team. POGIL is based on research indica ...
Document
... Woodward and the Fiesers performed extensive studies of terpene and steroidal alkenes and noted similar substituents and structural features would predictably lead to an empirical prediction of the wavelength for the lowest energy p p* electronic transition This work was distilled by Scott in 1964 ...
... Woodward and the Fiesers performed extensive studies of terpene and steroidal alkenes and noted similar substituents and structural features would predictably lead to an empirical prediction of the wavelength for the lowest energy p p* electronic transition This work was distilled by Scott in 1964 ...
Basic IUPAC Nomenclature V
... attached to the alcohol. This is now the main chain. Name this chain as if it were a straight chain alkane. a. Drop the –ne ending and add –ol. This works for –enes and – ynes as well. 2) Count the carbons in the main chain left to right and right to left. The direction you first run into the carbon ...
... attached to the alcohol. This is now the main chain. Name this chain as if it were a straight chain alkane. a. Drop the –ne ending and add –ol. This works for –enes and – ynes as well. 2) Count the carbons in the main chain left to right and right to left. The direction you first run into the carbon ...
D:\MyFiles\general manual\techniques\recrystallization.wpd
... molecules of Y, and X will be added preferentially. Thus, crystals of pure X will form, and excess Y is left behind in solution. Recrystallizing from solvent mixture A-B Great in theory, but how does it work in practise? The easiest way of recrystallizing a compound is when the compound is very solu ...
... molecules of Y, and X will be added preferentially. Thus, crystals of pure X will form, and excess Y is left behind in solution. Recrystallizing from solvent mixture A-B Great in theory, but how does it work in practise? The easiest way of recrystallizing a compound is when the compound is very solu ...
Homoaromaticity

Homoaromaticity in organic chemistry refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.The concept of homoaromaticity was pioneered by Saul Winstein in 1959, prompted by his studies of the “tris-homocyclopropenyl” cation. Since the publication of Winstein's paper, much research has been devoted to understanding and classifying these molecules, which represent an additional “class” of aromatic molecules included under the continuously broadening definition of aromaticity. To date, homoaromatic compounds are known to exist as cationic and anionic species, and some studies support the existence of neutral homoaromatic molecules, though these are less common. The 'homotropylium' cation (C8H9+) is perhaps the best studied example of a homoaromatic compound.