Department of Chemistry Second Year Syllabus
... Department of Chemistry – Imperial College INTRODUCTION TO SECOND YEAR CHEMISTRY Aims The second year of the degree in Chemistry aims to provide the students with an expanded and deeper understanding of the fundamental concepts required to rationalise and predict chemical reactivity. To achieve thi ...
... Department of Chemistry – Imperial College INTRODUCTION TO SECOND YEAR CHEMISTRY Aims The second year of the degree in Chemistry aims to provide the students with an expanded and deeper understanding of the fundamental concepts required to rationalise and predict chemical reactivity. To achieve thi ...
Document
... towards the X-H bond was included). A radial scaling for the spherical density was refined for each atom-type together with a scaling for the radial function for the deformation density (κ and κ’ were constrained to be equal to avoid divergence). It is known that non centrosymmetric structures might ...
... towards the X-H bond was included). A radial scaling for the spherical density was refined for each atom-type together with a scaling for the radial function for the deformation density (κ and κ’ were constrained to be equal to avoid divergence). It is known that non centrosymmetric structures might ...
PHYSICOCHEMICAL PROPERTIES OF ORGANIC MEDICINAL
... To understand the role of electronic effects in the acidity of various organic compounds more broadly, it is of value to compare the pKas of “typical” alcohols, phenols and carboxylic acids. Generally carboxylic acids are more acidic (pKas 3-5) than phenols (pKas 7-10) which are more acidic than alc ...
... To understand the role of electronic effects in the acidity of various organic compounds more broadly, it is of value to compare the pKas of “typical” alcohols, phenols and carboxylic acids. Generally carboxylic acids are more acidic (pKas 3-5) than phenols (pKas 7-10) which are more acidic than alc ...
Bromine
... radioactive isomer, with a half-life of 4.86 seconds. It decays by isomeric transition to the stable ground state. ...
... radioactive isomer, with a half-life of 4.86 seconds. It decays by isomeric transition to the stable ground state. ...
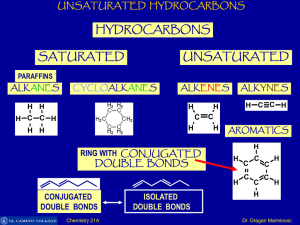
Unsaturated Hydrocarbons
... Benzene boils at 80°C - rather higher than other hydrocarbons of similar molecular size (pentane and hexane, for example). This is presumably due to the ease with which temporary dipoles can be set up involving the delocalized electrons. Methylbenzene (toluene) boils at 111°C. It is a bigger molecul ...
... Benzene boils at 80°C - rather higher than other hydrocarbons of similar molecular size (pentane and hexane, for example). This is presumably due to the ease with which temporary dipoles can be set up involving the delocalized electrons. Methylbenzene (toluene) boils at 111°C. It is a bigger molecul ...
What is a mole? - Chemical Paradigms
... and dissolving it in a known volume of water. More about this later. In a chemical reaction substances need to combine in the correct mole ratio in order for the reaction to occur. Even if the number of moles changes the ratio of moles of reactants and products does not change. In the reaction below ...
... and dissolving it in a known volume of water. More about this later. In a chemical reaction substances need to combine in the correct mole ratio in order for the reaction to occur. Even if the number of moles changes the ratio of moles of reactants and products does not change. In the reaction below ...
Chapter 23 - Simpson County Schools
... Aldehydes oxidize readily to carboxylic acids. Ketones can’t be oxidized as readily. ...
... Aldehydes oxidize readily to carboxylic acids. Ketones can’t be oxidized as readily. ...
Chapter 25 Organic and Biological Chemistry
... Reactions of Aromatic Compounds • Unlike in alkenes and alkynes, electrons do not sit between two atoms. • Electrons are delocalized; this stabilizes aromatic compounds. Organic and Biological Chemistry ...
... Reactions of Aromatic Compounds • Unlike in alkenes and alkynes, electrons do not sit between two atoms. • Electrons are delocalized; this stabilizes aromatic compounds. Organic and Biological Chemistry ...
Chapter 25 Organic and Biological Chemistry
... Reactions of Aromatic Compounds • Unlike in alkenes and alkynes, electrons do not sit between two atoms. • Electrons are delocalized; this stabilizes aromatic compounds. Organic and Biological Chemistry ...
... Reactions of Aromatic Compounds • Unlike in alkenes and alkynes, electrons do not sit between two atoms. • Electrons are delocalized; this stabilizes aromatic compounds. Organic and Biological Chemistry ...
Chapter 1 Review Questions
... cancerous. Although this evidence is consistent with the possible effects of long-term arsenic poisoning, it is not conclusive. Fortunately, Napoleon’s staff kept locks of his hair, which were then passed down within these families through generations. Eventually, modern forensic techniques were app ...
... cancerous. Although this evidence is consistent with the possible effects of long-term arsenic poisoning, it is not conclusive. Fortunately, Napoleon’s staff kept locks of his hair, which were then passed down within these families through generations. Eventually, modern forensic techniques were app ...
Review Graphite | SpringerLink
... It should be noted that pyrolytic graphite available before ∼1960 was not highly-oriented, so that experimental results on such materials should be treated with caution. 3. Bonding An isolated carbon atom has an electronic configuration of 1s2 2s2 2p2 . The 1s2 electrons belong to the ion core and t ...
... It should be noted that pyrolytic graphite available before ∼1960 was not highly-oriented, so that experimental results on such materials should be treated with caution. 3. Bonding An isolated carbon atom has an electronic configuration of 1s2 2s2 2p2 . The 1s2 electrons belong to the ion core and t ...
CHAPTER 15
... (1) In the IUPAC nomenclature system, an aldehyde group has priority over a ketone group. (2) Addition of an alcohol molecule across the carbon-oxygen double bond of an aldehyde produces a compound in which the carbonyl carbon atom bears both an alkoxy group and a hydroxy group. (3) 2-Propenal conta ...
... (1) In the IUPAC nomenclature system, an aldehyde group has priority over a ketone group. (2) Addition of an alcohol molecule across the carbon-oxygen double bond of an aldehyde produces a compound in which the carbonyl carbon atom bears both an alkoxy group and a hydroxy group. (3) 2-Propenal conta ...
Expression and Amplification of Chirality in Two
... bisuccinate on Cu(110).[23,24] Because the probability of generating both enantiomorphous lattices is identical, two-dimensional conglomerates are formed, i.e. all molecules in a single domain of the adlattice have the same chirality. As in the case of racemic bitartrate, both mirror structures are ...
... bisuccinate on Cu(110).[23,24] Because the probability of generating both enantiomorphous lattices is identical, two-dimensional conglomerates are formed, i.e. all molecules in a single domain of the adlattice have the same chirality. As in the case of racemic bitartrate, both mirror structures are ...
Chapter Twenty-four
... • The branch of chemistry that deals with carbon compounds is organic chemistry. • Classes of organic compounds can be distinguished according to functional groups they contain. • A functional group is a group of atoms that is largely responsible for the chemical behavior of the parent molecule. • M ...
... • The branch of chemistry that deals with carbon compounds is organic chemistry. • Classes of organic compounds can be distinguished according to functional groups they contain. • A functional group is a group of atoms that is largely responsible for the chemical behavior of the parent molecule. • M ...
Functional Group Isomerism
... Stereoisomers may possess quite different physical properties, such as melting point, density and solubility in water. Ring structures and other steric factors also result in geometric isomerism. ...
... Stereoisomers may possess quite different physical properties, such as melting point, density and solubility in water. Ring structures and other steric factors also result in geometric isomerism. ...
No Slide Title
... 3-6 Solving Compound Inequalities Every solution of a compound inequality involving AND must be a solution of both parts of the compound inequality. If no numbers are solutions of both simple inequalities, then the compound inequality has no solutions. The solutions of a compound inequality involvi ...
... 3-6 Solving Compound Inequalities Every solution of a compound inequality involving AND must be a solution of both parts of the compound inequality. If no numbers are solutions of both simple inequalities, then the compound inequality has no solutions. The solutions of a compound inequality involvi ...
Correlated/non-correlated ion dynamics of charge
... attention in the arena of molecular dynamics (MD), usually employing static high field approaches that probe fast motions.9–11 Our interest in this particular salt, which possesses limited stability under certain conditions,12 arises solely from the ionic symmetries provided, which are otherwise una ...
... attention in the arena of molecular dynamics (MD), usually employing static high field approaches that probe fast motions.9–11 Our interest in this particular salt, which possesses limited stability under certain conditions,12 arises solely from the ionic symmetries provided, which are otherwise una ...
Document
... The empirical formula of a compound is CH2 Which molecular formula is correctly paired with a structural formula for this compound? ...
... The empirical formula of a compound is CH2 Which molecular formula is correctly paired with a structural formula for this compound? ...
Lecture 1 Homework answers
... atom will have a ____ 6. When two atoms of different electronegativity form a covalent bond, the majority of shared electron more density is found around the ____________ electronegative atom. 7. For the following molecular formulas, draw complete Lewis line structures in which all atoms (even H ato ...
... atom will have a ____ 6. When two atoms of different electronegativity form a covalent bond, the majority of shared electron more density is found around the ____________ electronegative atom. 7. For the following molecular formulas, draw complete Lewis line structures in which all atoms (even H ato ...
Relative Atomic Masses
... Molecular Theory. Observation 1: Mass Relationships during Chemical Reactions Since matter is anything that has mass, then the Law of Conservation of Mass suggests that matter is also conserved, aka the Law of Conservation of Matter. During chemical reactions: whatever we start with, we wind up with ...
... Molecular Theory. Observation 1: Mass Relationships during Chemical Reactions Since matter is anything that has mass, then the Law of Conservation of Mass suggests that matter is also conserved, aka the Law of Conservation of Matter. During chemical reactions: whatever we start with, we wind up with ...
HOMOLOGATION OF HETEROCYCLES BY A SEQUENTIAL REDUCTIVE OPENING LITHIATION – S
... 04CRV2667) are of great interest in organic synthesis because polyfunctionalized molecules are obtained in a single synthetic operation by reaction with electrophilic reagents.(95MI5, 02MI6) Functionalized organolithium compounds can be prepared by halogen-lithium exchange, metal-lithium exchange, d ...
... 04CRV2667) are of great interest in organic synthesis because polyfunctionalized molecules are obtained in a single synthetic operation by reaction with electrophilic reagents.(95MI5, 02MI6) Functionalized organolithium compounds can be prepared by halogen-lithium exchange, metal-lithium exchange, d ...
Homoaromaticity

Homoaromaticity in organic chemistry refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.The concept of homoaromaticity was pioneered by Saul Winstein in 1959, prompted by his studies of the “tris-homocyclopropenyl” cation. Since the publication of Winstein's paper, much research has been devoted to understanding and classifying these molecules, which represent an additional “class” of aromatic molecules included under the continuously broadening definition of aromaticity. To date, homoaromatic compounds are known to exist as cationic and anionic species, and some studies support the existence of neutral homoaromatic molecules, though these are less common. The 'homotropylium' cation (C8H9+) is perhaps the best studied example of a homoaromatic compound.