Thiobenzoate Photochemistry
... While the elimination occurs with compounds possessing a -bond or heteroatom at the carbon, it fails with saturated hydrocarbons. The success of the reaction depends on the strength of the -C-H bond. For example, the quantum yields for the disappearance of Ph(C=S)OCH2CH2R are 0.46, 0.12 and 0.01 ...
... While the elimination occurs with compounds possessing a -bond or heteroatom at the carbon, it fails with saturated hydrocarbons. The success of the reaction depends on the strength of the -C-H bond. For example, the quantum yields for the disappearance of Ph(C=S)OCH2CH2R are 0.46, 0.12 and 0.01 ...
Experimental and Computational Evidence of Metal‑O2 Activation
... Initial rates of O2 consumption were determined with a Clarktype O2 (YSI) electrode. In contrast to the kinetic behavior of wild-type CuAGAO,60 CoAGAO exhibited a significant induction or lag phase. Maximal rates were estimated from the linear portion of time traces corresponding to the steepest slop ...
... Initial rates of O2 consumption were determined with a Clarktype O2 (YSI) electrode. In contrast to the kinetic behavior of wild-type CuAGAO,60 CoAGAO exhibited a significant induction or lag phase. Maximal rates were estimated from the linear portion of time traces corresponding to the steepest slop ...
Chem Outcomes - Hutchk12.org
... Use the concepts of dimensional analysis, scientific notation & significant figures to solve problems. Use the Metric (International) System (SI) to make measurements and in solving problems. Use dimensional analysis to solve problems and perform unit conversions. Use scientific notation to represen ...
... Use the concepts of dimensional analysis, scientific notation & significant figures to solve problems. Use the Metric (International) System (SI) to make measurements and in solving problems. Use dimensional analysis to solve problems and perform unit conversions. Use scientific notation to represen ...
Document
... Ch 9 Test: Chemical Quantities Round final answers to the correct number of significant figures. Balance all equations as necessary. Show work where indicated. 1. Given the balanced equation 2A + 3B 5C + 4D If 3.50 moles of A react, how many moles of product C can be formed? 2. Given the balanced ...
... Ch 9 Test: Chemical Quantities Round final answers to the correct number of significant figures. Balance all equations as necessary. Show work where indicated. 1. Given the balanced equation 2A + 3B 5C + 4D If 3.50 moles of A react, how many moles of product C can be formed? 2. Given the balanced ...
Chapter 20 Thermodynamics
... is the same even though they took very different paths to get to the mountain top. This is the power of all the state functions in thermodynamics---we only need to now the start and the finish bulk parameters. ...
... is the same even though they took very different paths to get to the mountain top. This is the power of all the state functions in thermodynamics---we only need to now the start and the finish bulk parameters. ...
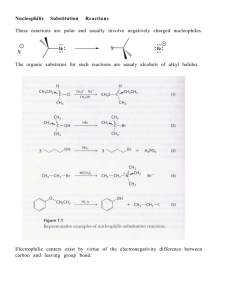
Chapter 8 I. Nucleophilic Substitution
... depend on the speed at which chloride smashes into methyl iodide. Data at lower collision energies support the traditional SN2 mechanism. However, at higher collision energies, about 10% of the iodide ions fell outside of the expected distribution. "We saw a group of iodide ions with a much slower v ...
... depend on the speed at which chloride smashes into methyl iodide. Data at lower collision energies support the traditional SN2 mechanism. However, at higher collision energies, about 10% of the iodide ions fell outside of the expected distribution. "We saw a group of iodide ions with a much slower v ...
Fundamentals of Organic Chemistry
... distinguish between an alkane, alkene, alkyne and an aromatic by structure. name each type of hydrocarbon by means of the IUPAC system. describe the physical properties of hydrocarbons and how these relate to biochemical properties. Use alkanes as model compounds to describe a. Constitutional isomer ...
... distinguish between an alkane, alkene, alkyne and an aromatic by structure. name each type of hydrocarbon by means of the IUPAC system. describe the physical properties of hydrocarbons and how these relate to biochemical properties. Use alkanes as model compounds to describe a. Constitutional isomer ...
Fundamentals of Organic Chemistry
... distinguish between an alkane, alkene, alkyne and an aromatic by structure. name each type of hydrocarbon by means of the IUPAC system. describe the physical properties of hydrocarbons and how these relate to biochemical properties. Use alkanes as model compounds to describe a. Constitutional isomer ...
... distinguish between an alkane, alkene, alkyne and an aromatic by structure. name each type of hydrocarbon by means of the IUPAC system. describe the physical properties of hydrocarbons and how these relate to biochemical properties. Use alkanes as model compounds to describe a. Constitutional isomer ...
... fraction of the enzymes; this fact may be due to the time used in the experimental procedure for dissolving (30 min) of the enzyme allowed just partial solution, in contrast longer stirring of the KAP in the aqueous reaction medium, during the whole reaction increased the dissolution of the enzyme. ...
纳米结构体系物理化学性质的理论研究方法与实例
... processes in which the same species participate in each reaction step. • The most common cases of parallel reactions are: (1) those in which the initial reactant decomposes into several different products; (2) those in which the initial reactants are different, but yield the same products; and (3) t ...
... processes in which the same species participate in each reaction step. • The most common cases of parallel reactions are: (1) those in which the initial reactant decomposes into several different products; (2) those in which the initial reactants are different, but yield the same products; and (3) t ...