Chem 3820 Outline - U of L Class Index
... E- mail: [email protected] (preferred) URL: http://people.uleth.ca/~p.hayes/ Phone: (403) 329-2313 Office Hours: 10:00 – 11:00 MWF (or by appointment) Class URL: http://classes.uleth.ca/200603/chem3820a/ Credit Hours: 3.0 Prerequisites: Chemistry 2600, 2720, 2810 Course Subject: The properties, struc ...
... E- mail: [email protected] (preferred) URL: http://people.uleth.ca/~p.hayes/ Phone: (403) 329-2313 Office Hours: 10:00 – 11:00 MWF (or by appointment) Class URL: http://classes.uleth.ca/200603/chem3820a/ Credit Hours: 3.0 Prerequisites: Chemistry 2600, 2720, 2810 Course Subject: The properties, struc ...
Chem 1202
... When a system undergoes a physical or chemical change, the change in its internal energy is equal to q, the heat energy flowing into or out of the system, plus w, the work (all other forms of energy) coming into or going out of the system: ...
... When a system undergoes a physical or chemical change, the change in its internal energy is equal to q, the heat energy flowing into or out of the system, plus w, the work (all other forms of energy) coming into or going out of the system: ...
Substrate scope of the re-engineered enzyme, FucO D93
... temperature and has to be experimentally determined. ...
... temperature and has to be experimentally determined. ...
Unit 2 Chemical Reactions
... Single Displacement reactions and the activity series ; reactivity of metals in water pg 127 ( 21 ) ; reactivity of metals with metallic ion solutions pg 131 ( 22 ) ; reactivity of non-metallics with non-metallic ions in solution. Pg 131 ( 23,24 ) ...
... Single Displacement reactions and the activity series ; reactivity of metals in water pg 127 ( 21 ) ; reactivity of metals with metallic ion solutions pg 131 ( 22 ) ; reactivity of non-metallics with non-metallic ions in solution. Pg 131 ( 23,24 ) ...
Chemistry S1 Unit 5 – Chemistry At Work Check for Understanding
... 5. What are half-reactions? A. Equations showing only the ions involved in a net ionic equation B. Equations showing what compounds are involved in a reaction C. Equations showing the electronegativities of atoms in a reaction D. Equations showing which elements are oxidized and which are reduced M ...
... 5. What are half-reactions? A. Equations showing only the ions involved in a net ionic equation B. Equations showing what compounds are involved in a reaction C. Equations showing the electronegativities of atoms in a reaction D. Equations showing which elements are oxidized and which are reduced M ...
Objective (Local, State, National – College Board)
... 1.a-b. reviewed from Chem I c. define terms, present resources such as table of electronegativities, link electronegativity to atom’s ability to draw an electron to it, describe through illustration how electronegativity differences help us to predict what kind of molecular bonds will form based on ...
... 1.a-b. reviewed from Chem I c. define terms, present resources such as table of electronegativities, link electronegativity to atom’s ability to draw an electron to it, describe through illustration how electronegativity differences help us to predict what kind of molecular bonds will form based on ...
T h - Website Staff UI
... Standard enthalpy of formation Standard enthalpy change for the formation of the compound from its elements in their reference states. Reference state of an element is its most stable state at the specified temperature & 1 bar ...
... Standard enthalpy of formation Standard enthalpy change for the formation of the compound from its elements in their reference states. Reference state of an element is its most stable state at the specified temperature & 1 bar ...
FACULTY OF PHARMACY Course Specifications: (CHEM 103)
... understand the theoretical basis of: Organic chemistry and the type organic chemical reactions involved. Methods of identification of aliphatic compounds. ii- Intellectual Skills By the end of this course the student should be able to; Write the structure and name of organic compounds. Draw the poss ...
... understand the theoretical basis of: Organic chemistry and the type organic chemical reactions involved. Methods of identification of aliphatic compounds. ii- Intellectual Skills By the end of this course the student should be able to; Write the structure and name of organic compounds. Draw the poss ...
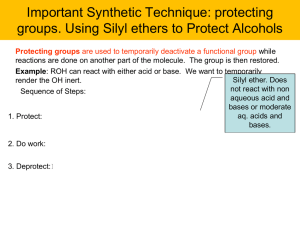
Spring 2015 CH 421 Name ________________________________________ Section ___________ Post‐lab 3: The Grignard Reaction: Preparation of an Alcohol
... Many aldehydes are prone to air oxidation. For instance, a bottle of benzaldehyde will turn from a clear liquid to a white solid if left open over time. What is the oxidation product generated in this case? ...
... Many aldehydes are prone to air oxidation. For instance, a bottle of benzaldehyde will turn from a clear liquid to a white solid if left open over time. What is the oxidation product generated in this case? ...
Heat
... The combustion reaction for a substance is defined as the reaction of one mole of a single substance with O2(g) to form combustion products. Because of the way in which we have defined the combustion reaction we may have to use fractional coefficients for some of the reactants and products. The enth ...
... The combustion reaction for a substance is defined as the reaction of one mole of a single substance with O2(g) to form combustion products. Because of the way in which we have defined the combustion reaction we may have to use fractional coefficients for some of the reactants and products. The enth ...