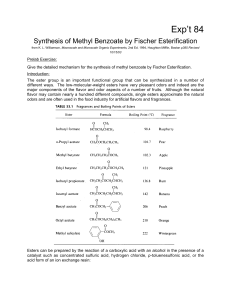
Chapter 8
... • List three observations that suggest that a chemical reaction has taken place. • List three requirements for a correctly written chemical equation. • Write a word equation and a formula equation for a given chemical reaction. • Balance a formula equation by inspection. ...
... • List three observations that suggest that a chemical reaction has taken place. • List three requirements for a correctly written chemical equation. • Write a word equation and a formula equation for a given chemical reaction. • Balance a formula equation by inspection. ...
экзаменационные тесты по органической химии
... 83. The molar mass of H3PO4 is: a. 8.00 g b. 47.98 g c. 82.00 g d. 98.00 g 84. Equation: Zn + 2HCl → ZnCl2 + H2↑ From the indicated equation, if 5.0 moles of zinc are used with excess HCl, how many moles of the product ZnCl2 would be formed. a. 1.0 b. 2.5 c. 5.0 d. 10.0 85. Equation: Zn + 2HCl → ZnC ...
... 83. The molar mass of H3PO4 is: a. 8.00 g b. 47.98 g c. 82.00 g d. 98.00 g 84. Equation: Zn + 2HCl → ZnCl2 + H2↑ From the indicated equation, if 5.0 moles of zinc are used with excess HCl, how many moles of the product ZnCl2 would be formed. a. 1.0 b. 2.5 c. 5.0 d. 10.0 85. Equation: Zn + 2HCl → ZnC ...
Chapter 9 Stoichiometry
... Percent yield tells us how “efficient” a reaction is. Percent yield can not be bigger than 100 %. ...
... Percent yield tells us how “efficient” a reaction is. Percent yield can not be bigger than 100 %. ...
PHT-224 Lectures 6
... Special Case Apparent zero order of reaction In aqueous suspensions of drugs, as the dissolved drug decomposes more drug dissolve to maintain drugconcentration i.e. drug concentration kept constant, once all undissolved drug is dissolved, rate becomes first order. ...
... Special Case Apparent zero order of reaction In aqueous suspensions of drugs, as the dissolved drug decomposes more drug dissolve to maintain drugconcentration i.e. drug concentration kept constant, once all undissolved drug is dissolved, rate becomes first order. ...
Alcohol oxidation
... A very interesting characteristic of this process is that it does not require any activation by a catalyst. The Hydroboration mechanism has the elements of both hydrogenation and electrophilic addition and it is a stereospecific (syn addition), meaning that the hydroboration takes place on the same ...
... A very interesting characteristic of this process is that it does not require any activation by a catalyst. The Hydroboration mechanism has the elements of both hydrogenation and electrophilic addition and it is a stereospecific (syn addition), meaning that the hydroboration takes place on the same ...
Alkanes CH4 + Cl2 → CH3Cl + HCl CH3CH3 + Cl2 → CH3CH2Cl +
... –Hal bonds. The reagents and conditions are to mix the alkane with bromine or chlorine and expose to ultra-violet light. The ultra-violet light provides enough energy to break the halogen bond, which forms species called free radicals. When the Hal-Hal bond breaks, it does so with one electron from ...
... –Hal bonds. The reagents and conditions are to mix the alkane with bromine or chlorine and expose to ultra-violet light. The ultra-violet light provides enough energy to break the halogen bond, which forms species called free radicals. When the Hal-Hal bond breaks, it does so with one electron from ...
CHEM 210 Ch06
... • A reaction coordinate is a variable that corresponds to the changes in geometry, on a molecular level, as reactants are converted into products. ...
... • A reaction coordinate is a variable that corresponds to the changes in geometry, on a molecular level, as reactants are converted into products. ...
CH 8 blackboard
... When 1 mole of C3H8 reacts with 5 moles of O2 to form 3 moles of CO2 and 4 moles of H2O, 2044 kJ of heat are emitted. These ratios can be used to construct conversion factors between amounts of reactants or products and the quantity of heat exchanged. ...
... When 1 mole of C3H8 reacts with 5 moles of O2 to form 3 moles of CO2 and 4 moles of H2O, 2044 kJ of heat are emitted. These ratios can be used to construct conversion factors between amounts of reactants or products and the quantity of heat exchanged. ...
Tech Info - Davis Instruments
... have to NMR as an analytical tool. While the technical aspects of executing the aldol reaction are not difficult, the analysis of products is challenging since this is frequently a student's first time using NMR instrumentation, interpreting NMR spectra and evaluating the outcome of their synthetic ...
... have to NMR as an analytical tool. While the technical aspects of executing the aldol reaction are not difficult, the analysis of products is challenging since this is frequently a student's first time using NMR instrumentation, interpreting NMR spectra and evaluating the outcome of their synthetic ...
Week - Syllabus | Chaminade
... intermediates and transition states where appropriate. Identify thermodynamically favorable conformations for acyclic and cyclic molecules Use principles of stereochemistry to explain stereoselective reactions Distinguish mechanisms on the basis of stereochemical outcome Improve spatial skil ...
... intermediates and transition states where appropriate. Identify thermodynamically favorable conformations for acyclic and cyclic molecules Use principles of stereochemistry to explain stereoselective reactions Distinguish mechanisms on the basis of stereochemical outcome Improve spatial skil ...
chapter10-bur.320702..
... The negative sign in the above equation occurs because we are measuring the value of q for the surroundings, and qsys = - qsur. If we know the energy of combustion for a compound, in units of kJ/g, then we can say q = m Ucom m = mass of compound burned Ucom = energy of combustion (in kJ/g) Note th ...
... The negative sign in the above equation occurs because we are measuring the value of q for the surroundings, and qsys = - qsur. If we know the energy of combustion for a compound, in units of kJ/g, then we can say q = m Ucom m = mass of compound burned Ucom = energy of combustion (in kJ/g) Note th ...
...detail
... 4. Polar and nonpolar character of carbon-carbon and carbon-heteroatom bonds. Qualitative idea of dipole moment and its application in aliphatic and aromatic compounds. Inter- and intramolecular forces and their effects on physical and chemical properties of molecules. (6 lectures) 5. Homolysis and ...
... 4. Polar and nonpolar character of carbon-carbon and carbon-heteroatom bonds. Qualitative idea of dipole moment and its application in aliphatic and aromatic compounds. Inter- and intramolecular forces and their effects on physical and chemical properties of molecules. (6 lectures) 5. Homolysis and ...
Orbitals - drjosephryan.com
... Conjugate Nucleophilic Addition to α,βUnsaturated Aldehydes and Ketones Conjugated double bond of a,b-unsaturated carbonyl is activated by carbonyl group of the aldehyde or ketone • C=C double bond is not activated for addition in absence of carbonyl group ...
... Conjugate Nucleophilic Addition to α,βUnsaturated Aldehydes and Ketones Conjugated double bond of a,b-unsaturated carbonyl is activated by carbonyl group of the aldehyde or ketone • C=C double bond is not activated for addition in absence of carbonyl group ...
Reaction Mechanisms
... Nucleophilic Attack on Coordinated Ligands Many different kinds of examples of this. From our prespective the more important ones involve attack on M–CO complexes and M–alkene/alkyne complexes. Attack on M–C π-Bonds – By ligating the metal, alkenes and alkynes usually become electrophilic. This mak ...
... Nucleophilic Attack on Coordinated Ligands Many different kinds of examples of this. From our prespective the more important ones involve attack on M–CO complexes and M–alkene/alkyne complexes. Attack on M–C π-Bonds – By ligating the metal, alkenes and alkynes usually become electrophilic. This mak ...