Bond Dissociation Energies of Organic Molecules
... In eq 16, “[thermal correction]” is a set of heat capacity integrals, ∫[Cp(R) - Cp(R-) + Cp(H) - Cp(H+)] dT, which may be evaluated as previously described, but their value is generally less than 0.3 kcal mol-1. By rearranging eq 16, one can estimate typical values for ∆acidH298(RH); since DH298(RH) ...
... In eq 16, “[thermal correction]” is a set of heat capacity integrals, ∫[Cp(R) - Cp(R-) + Cp(H) - Cp(H+)] dT, which may be evaluated as previously described, but their value is generally less than 0.3 kcal mol-1. By rearranging eq 16, one can estimate typical values for ∆acidH298(RH); since DH298(RH) ...
Ethers and Epoxides
... Thiols: Formation and Reaction From alkyl halides by displacement with a sulfur ...
... Thiols: Formation and Reaction From alkyl halides by displacement with a sulfur ...
Ethers and Epoxides - faculty at Chemeketa
... nucleophile such as –SH The alkylthiol product can undergo further reaction with the alkyl halide to give a symmetrical sulfide, giving a poorer yield of the thiol ...
... nucleophile such as –SH The alkylthiol product can undergo further reaction with the alkyl halide to give a symmetrical sulfide, giving a poorer yield of the thiol ...
Lecture Notes in Physical Chemistry Semester 2: Kinetics and
... kinetic energy of the molecule to kT , the average energy available at temperature T. What do these curves look like? The normalization part does not depend on v; the v 2 part is a parabola; the Boltzmann part is a Gaussian centered at zero. So at low speeds the curve looks like a rising parabola, t ...
... kinetic energy of the molecule to kT , the average energy available at temperature T. What do these curves look like? The normalization part does not depend on v; the v 2 part is a parabola; the Boltzmann part is a Gaussian centered at zero. So at low speeds the curve looks like a rising parabola, t ...
Chapter 18 – Carbonyl Compounds II (Last Chapter we mostly talk
... nucleophile. This reaction was first discovered in the 1800’s by Hugo Schiff a German Chemist, and the product of this reaction is often referred to as a Schiff base). *** ppt *** Fill in the Reagent of Product in the Box ...
... nucleophile. This reaction was first discovered in the 1800’s by Hugo Schiff a German Chemist, and the product of this reaction is often referred to as a Schiff base). *** ppt *** Fill in the Reagent of Product in the Box ...
Chapter 11
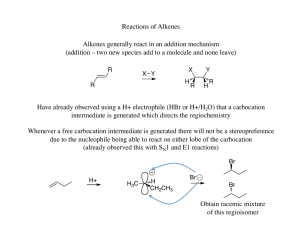
... Experimentally it is known, however, that rearrangements do nor occur with Br2 addition ...
... Experimentally it is known, however, that rearrangements do nor occur with Br2 addition ...
Thermodynamics The First Law Work, Heat, Energy
... The enthalpy of formation is the enthalpy change when a compound is formed from its elements, and those elements are in their most stable form under the prevailing conditions. When the prevailing conditions are the standard state, this is called the standard enthalpy of formation, ∆Hof ...
... The enthalpy of formation is the enthalpy change when a compound is formed from its elements, and those elements are in their most stable form under the prevailing conditions. When the prevailing conditions are the standard state, this is called the standard enthalpy of formation, ∆Hof ...
Manganese-Catalyzed Carbonylation of Alkyl Iodides
... variety of nucleophiles was developed. The method concerns the alkoxy and amino carbonylation as well as the use of more unconventional nucleophiles such as thiols, azide and hydride. The method employs alkyl iodides although bromides are also feasible substrates through in situ Finkelstein reaction ...
... variety of nucleophiles was developed. The method concerns the alkoxy and amino carbonylation as well as the use of more unconventional nucleophiles such as thiols, azide and hydride. The method employs alkyl iodides although bromides are also feasible substrates through in situ Finkelstein reaction ...