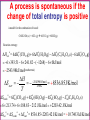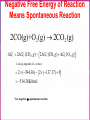* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Electrochemistry wikipedia , lookup
Eigenstate thermalization hypothesis wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Temperature wikipedia , lookup
Marcus theory wikipedia , lookup
Rubber elasticity wikipedia , lookup
Heat transfer physics wikipedia , lookup
George S. Hammond wikipedia , lookup
Glass transition wikipedia , lookup
Electrolysis of water wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Work (thermodynamics) wikipedia , lookup
Thermodynamics wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Transition state theory wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
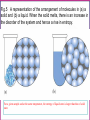
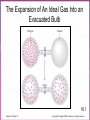
Chapter Sixteen: SPONTANEITY, ENTROPY, AND FREE ENERGY 講義 Assignment • 1-100題裡每10題中挑選1-2題完成。 Chapter 16 | Slide 2 Copyright © Houghton Mifflin Company. All rights reserved. • 本章投影片做為課前課後參考。 • 在課堂講解的投影片主要採用Atkins書的第 17章: http://140.117.34.2/faculty/phy/sw_ding/teaching/gchem/gc17.ppt Chapter 16 | Slide 3 Copyright © Houghton Mifflin Company. All rights reserved. The Big Question Why do some changes take place? What parameter(s) control or drive a change? • Energy (loss or gain) is required—obvious! • How energy is distributed also affects the tendency of a change—not that obvious! • Both entropy and enthalpy are important to drive a change although some processes are primarily driven by enthalpy while others by entropy. Overall, the Gibbs free energy is the parameter that controls the possibility of a change. We’ll find how this conclusion is drawn. Spontaneous Change A change that tends to occur without needing to be driven by an external influence. Fig.1 The direction of spontaneous change is for a hot block of metal (left) to cool to the temperature of its surroundings (right). A block at the same temperature as its surroundings does not spontaneously become hotter. Fig.2 The direction of spontaneous change for a gas is toward filling its container. A gas that already fills its container does not collect spontaneously in a small region of the container. A glass cylinder containing a brown gas (upper piece of glassware in the left illustration) is attached to an empty flask. When the stopcock between them is opened, the brown gas fills both upper and lower vessels (right illustration). The brown gas is nitrogen dioxide. NO2 Fig.3 We can understand the natural direction of the migration of heat from a hot region to a cold region by thinking about the jostling between the vigorously moving atoms in the hot region. Molecules jostle their neighbors, and the thermal motion spreads. Spontaneity—the spreading of energy to more and more degrees of freedom. Entropy is the measure of the number of degrees of freedom affected by thermal motion. Fig.4 We can understand the natural direction of the migration of matter by visualizing how the random motion of molecules results in their spreading throughout the available space. Spontaneity—the spreading of energy to more and more degrees of freedom. Entropy is the measure of the number of degrees of freedom affected by thermal motion. The second law of thermodynamics: The entropy of an isolated system tends to increase. Fig.5 A representation of the arrangement of molecules in (a) a solid and (b) a liquid. When the solid melts, there is an increase in the disorder of the system and hence a rise in entropy. For a given sample and at the same temperature, the entropy of liquid state is larger than that of solid state. Fig.6 The entropy of a solid increase as its temperature is raised. The entropy increase sharply when the solid melts to form the more disordered liquid and then gradually increases again up to the boiling point. A second, larger jump in entropy occurs when the liquid turns into a vapor. For a given sample, the entropy is larger at higher temperatures than that at lower temperatures. Example: predicting the relative entropies of two samples Which has the greater entropy: (a) 1 g of pure solid NaCl or 1 g of NaCl o dissolved o in 100 mL of water; (b) 1 g of water at 25 C or a 1 g of water at 50 C? Ans: • • 1 g of NaCl dissolved o in 100 mL of water. 1 g of water at 50 C. For a given sample and at the same temperature, the entropy of liquid state is larger than that of solid state. For a given sample, the entropy is larger at higher temperatures than that at lower temperatures. Exercise: predicting the relative entropies of two samples Which has the greater entropy: 1 mol of CO2 (s) or 1 mol of CO2 (g) at the same temperature? Ans: 1 mol of CO2 (g) o Which has the greater entropy: a sample of liquid mercury at -15 C o or the same sample at 0 C? o Ans: The sample of liquid mercury at 0 C. Entropy: Macroscopic Definition and Calculation heat supplied reversibly Change in entropy = temperature at which the transfer takes place qrev dq S , unit: J/K T T rev A reversible process is one that can be reversed by an infinitesimal change in a variable. (when heat is transferred to a system, the source must have the same temperature as the system itself.) The greater the energy transferred to the system as heat, the greater the increase in entropy. If the transfer is made to a hot system, the increase in entropy is smaller than when the same amount of energy is transferred to a cool system. Calculating the Change in Entropy Calculate the change in entropy of a large tank of water when a total o of 100.0 J of energy is transferred to it reversibly as heat at 20.0 C. qrev S T 100.0J 293 K =+0.341 J/K o Calculate the change in entropy of a large iron block at 20.0 C when 1500.0 J of energy escapes as heat from the block to the surroundings. qrev S T 1500.0J 297 K =-5.05 J/K Classroom Exercise: Calculating the Change in Entropy o Calculate the change in entropy of a large swimming pool at 28.0 C when 240.0 J of escapes from the pool as heat to the surroundings. qrev S T 240.0J 299 K =-0.803 J/K Fusion and vaporization entropies The discontinuities correspond to phase transition. The jump of entropy is the signature of first order phase transition. Calculating the Entropy of Vaporization Calculate the change in molar entropy when water vaporizes at its boiling point. Svap qrev H vap Tb Tb 40.7kJ/mol 373 K =+109 J/K/mol Calculating the Entropy of Fusion Calculate the change in molar entropy of ice at its melting point. (Look Table 6.2 for fusion enthalpy) S fus qrev H fus Tm Tm 6.06kJ/mol 273 K =+22.0 J/K/mol Classroom Exercise: Calculating the Entropy of Vaporization Calculate the change in molar entropy when ammonia vaporizes at its boiling point (239.7 K). The vaporization enthalpy of ammonia is 23.4 kJ/mol. Svap qrev H vap Tb Tb 23.4kJ/mol 239.7 K =+97.62 J/K/mol Entropy: Microscopic Definition and Calculation Entropy = The number of microscopic states for a given macroscopic state S k ln W k: Boltzmann constant Entropy is a measure of how the energy of a system is stored for a given macroscopic state. Absolute Entropies • The third law of thermodynamics: The entropy of a perfect crystal approaches 0 as the absolute temperature approaches 0. • All absolute entropies are positive Standard molar entropy Smo (298 K). More complicated compounds have higher entropies Entropies are higher at higher temperatures You take more entropy after you drink a cup of hot tea than a cup of iced tea. You take more entropy when you’re in fever than when you’re normal after you drink a cup of tea. Fig.7 The entropy change due to heat transfer depends on both the amount of heat transferred and the temperature of the system. A lot of heat transferred to a cold system (upper left) results in a large increase in the entropy of the system. A small quantity of heat transferred to a hot system (lower right) results in a small increase in entropy of the system. The entropy is higher for • • • • • Higher temperature Larger volume More complex structures Larger sample size Heavier atoms (entropy is not disorder!) • Vapor relative to liquid or solid • Liquid relative to solid Estimating the relative value of the molar entropy Which substance in each pair has the higher molar entropy: (a) CO2 at 25 oC and 1 atm or CO2 at 25 oC and 3 atm; (b) Br2(l) or Br2(g) at the same temperature and pressure; (c) methane gas, CH4, or propane gas, CH3CH2CH3 at the same temperature and pressure? o (a) oOne mole of CO2 at 25 C and 1 atm occupies larger volume than one mole of CO2 at 25 o C and 3 atm One mole of CO2 at 25 C and 1 atm has the higher molar entropy. (b) Gas has the higher molar entropy than liquid. Therefore, Br2(g) has the higher molar entropy at the same temperature and pressure. (c) One mole of CH4 is lighter than one mole of CH3CH2CH3 . Therefore, CH3CH2CH3 has the higher molar entropy at the same temperature and pressure. Exercise Which substance in each pair has the higher molar entropy: (a) He at 25 oC or He at 100 oC in a container of the same volume; (b) Br(g) or Br2(g) at the same temperature and pressure? The higherothe temperature, the higher the molar entropy o He at 100 C has the higher molar entropy than He at 25 C in a container of the same volume. The heavier the molecule/atom, the higher the molar entropy Br2(g) has the higher molar entropy than Br(g) at the same temperature and pressure. Classroom Exercise Which substance in each pair has the higher molar entropy at the same temperature and pressure : (a) Pb(s) or Pb(l); (b) SbCl3(g) or SbCl5(g) ? Liquid has the higher molar entropy than solid. Therefore, Pb(l) has the higher molar entropy than Pb(s) at the same temperature and pressure. The heavier the molecule/atom, the higher the molar entropy SbCl5(g) has the higher molar entropy than SbCl3(g) at the same temperature and pressure. Reaction Entropy ΔSr o = nSmo (products) nSmo (reactants) The standard molar entropies of common compounds are listed in Appendix 2 Because the molar entropy of a gas is so much greater than that of Solids and liquids, a change in the amount of gas normally dominates any other entropy change in a reaction. A net consumption of gas usually results in a negative reaction entropy. A net production of gas usually results in a positive reaction entropy. N2(g) + 3H2 (g) 2NH3 (g) Reactant: 4 mol, Product: 2 mol decrease in entropy. ΔSr o = 2Som (NH3 ,g) [Som (N 2 ,g) 3Som (H 2 ,g)] 2 192.4 (191.6 3 130.7) 198.9 J/K/mol (2) Exercise N2O4 (g) 2NO2 (g) Reactant: 1 mol, Product: 2 mol increase in entropy. ΔSr o = 2Som (NO 2 ,g) Som (N 2O 4 ,g) 2 240.06 304.29 175.83 J/K/mol Classroom Exercise C2H4 (g) +H2(g) C2H6 (g) Reactant: 2 mol, Product: 1 mol decrease in entropy. ΔSr o = Som (C2 H6 ,g) [Som (H 2 ,g) Som (C2H 4 ,g)] 229.60 219.56 130.68 120.64 J/K/mol Why does ice freeze spontaneously? Why exothermic reactions occur spontaneously? Total entropy change =entropy change of system +entropy change of surroundings Stot S S surr (3) A process is spontaneous as long as the total entropy change is positive. A spontaneous process does NOT require the increase of the entropy of the system. Fig.8 (a) In an exothermic process, heat escapes into the surroundings and increases their entropy. (b) In an endothermic process, the entropy of the surroundings decreases. The blue-green arrows indicate the direction of entropy change in the surroundings. Entropy change of surroundings heat trandferred to surroundings = temperature of surroundings enthalpy change of system = temperature of surroundings S surr H T (4) Exothermic reactions occur spontaneously because the increase of the entropy of the surroundings is more than the decrease of the system. N2(g) + 3H2 (g) 2NH3 (g) ΔH r o = 2Hfo (NH3 ,g) [ H fo (N 2 ,g) 3H fo (H 2 ,g)] 2 (46.11) (0 3 0) kJ/mol 92.22 kJ/mol Standard reaction enthalpy = -92.22 kJ/mol < 0 exothermic The entropy of the surroundings increases. S surr H T 92220J/mol 298K 309J/K/mol Fig.9 In an exothermic reaction, (a) the overall entropy change is certainly positive when the entropy of the system increases. (b) The overall entropy change is positive even when the entropy of the system decreases, provided that the entropy increase in the surroundings is greater. The reaction is spontaneous in both cases. ΔS > 0,ΔSsurr > 0 ΔS 0,ΔSsurr > 0, ΔSsurr >| ΔS |, ΔStot = ΔS + ΔSsurr > 0 ΔStot = ΔS + ΔSsurr > 0 Fig.10 An endothermic reaction is spontaneous only when the entropy of the system increases enough to overcome the decrease in entropy of the surroundings, as it does here. ΔS 0,ΔSsurr 0,| ΔSsurr | ΔS, ΔStot = ΔS + ΔSsurr > 0 A process is spontaneous if the change of total entropy is positive Is the dissolution of ammonium nitrate to form a dilute aqueous o solution spontaneous at 25 C? + NH4NO3 (s) NH4 (aq) + NO3 (aq) Reaction entropy: o sol ΔH = H fo (NH 4+ ,aq) H fo (NO3- ,aq) H fo (NH 4 NO3 ,s) 135.21 205.0 (365.56)kJ/mol 28.0kJ/mol S surr (endothermic) H 28000J/mol 94J/K/mol 298K T ΔSsol o = Smo (NH +4 ,aq) Smo (NO3- ,aq) Smo (NH 4 NO3 ,s) 113.4 146.4 151.08 J/K/mol 108.7 J/K/mol ΔS tot o = ΔSsol o ΔSsurr o = 108.7-94.0 J/K/mol = +14.7 J/K/mol A process is spontaneous if the change of total entropy is positive A model for the combustion of wood: C6H12O6 (s) + 6O2 (g) 6CO2 (g) +6H2O(g) Reaction entropy: ΔH sol o = 6H fo (CO2 ,g) 6H fo (H 2O,g) H fo (C6H12O6 ,s) 6H fo (O 2 ,g) 6 393.51 6 241.82 (1268) 6 0kJ/mol 2543.98kJ/mol(exothermic) S surr H 2543980J/mol 8536.85J/K/mol 298K T ΔScomb o = 6Smo (CO2 ,g) 6Smo (6H 2O,g) 6 Smo (6O 2 ,g) Smo (C6H12O6 ,s) 6 213.74 6 188.83 212 J/K/mol 2203.42 J/K/mol ΔS tot o = ΔScomb o ΔSsurr o = 8536.85+2203.42 J/K/mol = +10.7403 kJ/K/mol Gibbs free energy must decrease for a spontaneous change: ΔStot>0ΔG = -TΔStot<0 Stot S S surr H Stot S T G T Stot H T S Free energy is effectively the same as the total entropy (except for the negative sign and coefficient T) Josiah Willard Gibbs (1839–1903). ΔG < 0 the process is spontaneous ΔG > 0 the reverse of the process is spontaneous ΔG = 0 both the process and its reverse are spontaneous equilibrium. Free energy must decrease for a spontaneous change: ΔG<0 Free energy and equilibrium At equilibrium, both directions are equally spontaneous, then G T Stot 0 This condition applies to any phase change and any chemical reaction at equilibrium at constant temperature and pressure. Example: water-ice equilibrium---- H freeze H fus 6.00 kJ/mol S freeze S fus 21.97 J/k mol= 21.97 103kJ/k mol G freeze H freeze T S freeze G freeze (6.00kJ/mol) (273.15k) ( 21.97 10 3kJ/k mol) =0.00kJ/mol Predicting the boiling point of a substance Liquid metals, such as mixtures of sodium and potassium, are used as coolants in some nuclear reactors. Predict the normal boiling point of liquid sodium, given that the standard entropy of vaporization of liquid sodium is 84.8 J/K/mol and that its standard enthalpy of vaporization is 98.0 kJ/mol At equilibrium, both directions are equally spontaneous, then G T Stot 0 H vap Tb Svap 0 Tb H vap Svap 98000J/mol Tb 84.8J/K/mol 1160K Predicting the melting point of a substance Predict the normal melting point of solid chlorine, given that the standard entropy of fusion is 837.3 J/K/mol and that its standard enthalpy of fusion is 6.41 kJ/mol. At equilibrium, both directions are equally spontaneous, then G T Stot 0 H fus Tm S fus 0 Tm H fus S fus 6410J/mol Tm 37.3 J/K/mol =172K Classroom Exercise: Predicting the boiling point of methanol Predict the normal boiling point of methanol, CH3OH, given that the standard entropy of vaporization is 104.7 J/K/mol and that its standard enthalpy of vaporization is 35.3 kJ/mol. Tb Tb 35300J/mol 104.7J/K/mol H vap Svap 337K=64 C o Case Study 17 (a) The bubbles on the leaves of this underwater plant are oxygen produced by photosynthesis. Molecules such as the chlorophyll that colors the leaves green capture sunlight to begin the transformation of carbon dioxide and water to glucose and oxygen. Case Study 17 (b) A weight with a small mass can be lifted into the air by another weight of the same or greater mass. What would appear unnatural if we saw it by itself (a weight rising) is actually part of a spontaneous event overall. The “natural” fall of the heavier weight causes the “unnatural” rise of the smaller weight. Standard Reaction Free Energies Gr H r T Sr ΔHr = nHf (products) nHf (reactants) o o o ΔSr = nSm (products) nSm (reactants) o o o ΔGr o = nGf o (products) nGf o (reactants) Standard free energies of formation The most stable form of an element is the state with lowest free energy of formation. G f minimized The most stable form of a compound is the state with lowest free energy of formation. G f minimized Standard Reaction Free Energies Gr H r T Sr o Calculate the standard free energy of formation of HI(g) at 25 C from Its standard entropy and standard enthalpy of formation. 1 1 H 2 (g) I 2 (s) HI(g) 2 2 1 1 H r H f (HI,g) H f (H 2 ,g) H f (I 2 ,s) 2 2 =H f (HI,g)=+26.48kJ/mol 1 1 Sr Sm (HI,g) Sm (H 2 ,g) Sm (I 2 ,s) 2 2 1 1 206.59 130.68 116.14J/K/mol 2 2 Gr H r T Sr =83.18J/K/mol 26.48 kJ/mol -298 K 0.08318 kJ/K/mol =1.69 kJ/mol = Gf (HI,g) More Exercise o Calculate the standard free energy of formation of NH3(g) at 25 C. 1 3 N 2 (g)+ H 2 (g) NH 3 (g) 2 2 1 3 H r H f (NH3 ,g) H f (H 2 ,g) H f (N 2 ,g) 2 2 =Hf (NH3 ,g)=-46.11 kJ/mol 1 3 Sr Sm (NH 3 ,g) Sm (H 2 ,g) Sm (N 2 ,g) 2 2 3 1 192.45 130.68 191.61J/K/mol 2 2 Gr H r T Sr =99.375 J/K/mol 46.11 kJ/mol -298 K 0.099375 kJ/K/mol =-16.5 kJ/mol = Gf (NH3 ,g) Classroom Exercise Calculate o the standard free energy of formation of cyclopropane, C3H6(g) at 25 C. 3C(s)+3H2 (g) C3H6 (g) Hr Hf (C3H6 ,g) 3Hf (H 2 ,g) 3H f (C,s) =20.42-3 0-3 716.68 kJ/mol=-2129.62 kJ/mol Sr Sm (C3H 6 ,g) 3Sm (H 2 ,g) 3Sm (C,s) 237.4 3 130.68 3 158.10J/K/mol =-628.94 J/K/mol Gr H r T Sr 2129.62 kJ/mol -298 K (0.62894) kJ/K/mol =-1942.1959 kJ/mol = Gf (C3H 6 ,g) Figure 17.12 The standard free energies of formation of compounds are defined as the standard reaction free energy for their formation from the elements. They represent a thermodynamic “altitude” with respect to the elements at “sea level.” The numerical values are in kilojoules per mole. Standard Free Energy of Formation Decides Thermodynamic Stability If the standard free energy of formation of a compound is smaller than 0, thermodynamically stable. , then it is G f 0 If the standard free energy of formation of a compound is larger than 0, thermodynamically unstable. then it is G f 0, Example: 6C(s)+3H 2 (g) C6H6 (l) G f 124 kJ/mol >0 Thermodynamically unstable C6H6 (l) 6C(s)+3H 2 (g) G f 124 kJ/mol<0 Thermodynamically stable Standard Free Energy of Formation Decides Thermodynamic Stability o Is glucose stable relative to its elements at 25 C and under standard conditions? C6H12O6 (s) 6C (s) + 6H2(g) + 3O2(g) Standard free energy of formation (From Appendix 2A—Page A13): ΔGf = Gfo (C6H12O6 ,s) 6Gfo (C,s) 6Gfo (H 2 ,g) 3Gfo (O2 ,g) 910.0 0 0 0kJ/mol 910kJ/mol<0 thermodynamically stable. o Is methylamine, CH3NH2, stable relative to its elements at 25 C and under standard conditions? Gfo (CH3 NH 2 ,g) 32.16kJ/mol > 0 thermodynamically unstable. Standard Free Energy of Formation Decides Thermodynamic Stability o Is methylamine, CH3NH2, stable relative to its elements at 25 C and under standard conditions? Look up Appendix 2A, page A13, and we find the standard free energy of formation of methylamine is 32.16 kJ/mol. Gfo (CH3 NH 2 ,g) 32.16kJ/mol > 0 thermodynamically unstable. The Chemical Reaction Proceeds So That The Free Energy of o Reaction ΔG r < 0. ΔGr = nΔGf (products) nΔGf (reactants) o ΔG r o o o 4NH3 (g)+5O2 (g) 4NO(g)+6H2O(g) = 4ΔG (NO,g)+6ΔG (H O,g) 4ΔG (NH ,g)+5ΔG (O ,g) o f o f o 2 f o 3 = 4 86.55+6 ( 228.57) 4 ( 16.45)+0 kJ/mol = 959.42 kJ/mol spontaneous reaction. f 2 Negative Free Energy of Reaction Means Spontaneous Reaction 2CO(g)+O2 (g) 2CO2 (g) Gr 2Gf (CO 2 , g ) 2Gf (CO, g ) Gf (O 2 , g ) Look up Appendix 2A, we have 2 (394.36) 2 (137.17) 0 514.38kJ/mol Very negative spontaneous reaction. Negative Free Energy of Reaction Means Spontaneous Reaction 2SO2 (g)+O2 (g) 2SO3 (g) Gr 2Gf (SO3 , g ) 2Gf ( SO2 , g ) Gf (O 2 , g ) Look up Appendix 2A, we have 2 (371.06) 2 (300.19) 0 141.74kJ/mol Negative spontaneous reaction. Negative Free Energy of Reaction Means Spontaneous Reaction 6CO2 (g)+6H2O(l) C6H12O6 ( s) 6O2 (g) Gr Gf (C6H12O6 , s) 6Gf (O2 , g ) 6Gf (CO2 ,g ) 6Gf (H 2O, l ) Look up Appendix 2A, we have 910 6 (394.36) 6 (237.13) 2878.94kJ/mol Very positive not spontaneous reaction (the reverse is). In biological systems, glucose is synthesized by assistance of a special bioenzyme. Reaction Free Energy Varies With Temperature ΔG r =ΔG r +RT 1n Q o RT=(8.314 51J/K mol) (298.15 K)=2.4790 kJ/mol at 298.15K At T 0 K, Q 0 (no reaction occurs), RTlnQ is generally not zero so ΔG r (0K) ΔG r o Reaction free energy can be determined from reaction quotient. Figure 17.13 At constant temperature and pressure, the direction of spontaneous change is toward lower free energy. The equilibrium composition of a reaction mixture corresponds to the lowest point on the curve. In this example, substantial quantities of both reactants and products are present at equilibrium, and K is close to 1. Figure 17.14 In this reaction, the free energy is a minimum when products are much more abundant than reactants. The equilibrium lies in favor of the products, and K 1. This reaction effectively goes almost to completion. Figure 17.15 In this reaction, the free energy is a minimum when the reactants are much more abundant than the products. The equilibrium lies in favor of the reactants, and K 1. This reaction “does not go.” Free Energy of Reaction Gives The Temperature Dependence of The Equilibrium Constant 0=ΔG r +RT lnK o ΔG r = RT lnK K e o K<1 when ΔG r >0 o K>1 when ΔG r <0 o Gr o - RT Equilibrium Constant Can Be Found From Free Energies o Calculate Kp at 25 C for the equilibrium N 2O4 (g) N 2O4 (g) 2NO2 (g) 2NO2 (g) K p PNO2 2 PN2O4 Gr 2Gf ( NO2 , g ) Gf ( N 2O4 , g ) Gr 4730J/mol ln K p 8.3145J/K/mol×298K 1.91 RT K p 0.15 Figure 17.16 A negative value of the standard reaction free energy corresponds to an equilibrium constant greater than 1 and to products (yellow) favored over reactants (purple) at equilibrium. A positive value of the standard reaction free energy corresponds to an equilibrium constant of less than 1 and to reactants favored over products at equilibrium. Estimate the minimum temperature at which K>1 K>1 when the reaction free energy becomes negative r r r G H T S 0 T H r Sr Objectives (1) Skills You Should Have Mastered • Conceptual 1. State and explain the implications of the second law of thermodynamics, Section 17.2. 2. Explain how temperature, volume, and state of matter affect the entropy of a substance, Sections 17.2 and 17.3. 3. Show how ΔSsurr is related to ΔH for a change at constant temperature and pressure and justify the relationship, Section 17.5. 4. Show how the free energy change accompanying a process is related to the direction of spontaneous reaction and the position of equilibrium, Sections 17.7 and 17.10. • Descriptive 1. Describe the criteria for spontaneity of a reaction, Sections 17.5 and 17.6. 2. Identify thermodynamically unstable compounds from their standard free energies of formation, Section 17.8. • Objectives (2) Problem-Solving 1. Predict which of two systems has the greater entropy, given their compositions and conditions, Toolbox 17.1 and Example 17.1. 2. Calculate the change in entropy of a system due to heat transfer and phase changes, Toolbox 17.1 and Examples 17.2 and 17.3. 3. Estimate the relative molar entropies of two substances, Toolbox 17.1 and Example 17.4. 4. Calculate the standard reaction entropy from standard molar entropies, Example 17.5. 5. Judge the spontaneity of a reaction from its standard reaction enthalpy and standard reaction entropy, Example 17.6. 6. Predict the boiling point and melting point of a substance from the changes in entropy and enthalpy of the substance, Example 17.7. 7. Calculate a standard free energy of formation from the standard enthalpy of formation and standard molar entropies, Example 17.8. 8. Calculate the standard reaction free energy from free energies of formation, Example 17.9. 9. Calculate the reaction free energy from ΔGr° and the reaction quotient, Example 17.10. 10. Calculate an equilibrium constant from ΔGr° at a given temperature, Toolbox 17.2 and Example 17.11. 11. Predict the temperature at which a process with known DH and DS becomes spontaneous, Example 17.12. Thermodynamics vs. Kinetics Chapter 16 | Slide 71 Copyright © Houghton Mifflin Company. All rights reserved. Spontaneous Processes and Entropy • Thermodynamics lets us predict whether a process will occur but gives no information about the amount of time required for the process. • A spontaneous process is one that occurs without outside intervention. 16.1 Chapter 16 | Slide 72 Copyright © Houghton Mifflin Company. All rights reserved. Concept Check • Consider 2.4 moles of a gas contained in a 4.0 L bulb at a constant temperature of 32°C. This bulb is connected by a valve to an evacuated 20.0 L bulb. Assume the temperature is constant. a) What should happen to the gas when you open the valve? 16.1 Chapter 16 | Slide 73 Copyright © Houghton Mifflin Company. All rights reserved. Concept Check • Consider 2.4 moles of a gas contained in a 4.0 L bulb at a constant temperature of 32°C. This bulb is connected by a valve to an evacuated 20.0 L bulb. Assume the temperature is constant. b) Calculate H, E, q, and w for the process you described above. 16.1 Chapter 16 | Slide 74 Copyright © Houghton Mifflin Company. All rights reserved. Concept Check • Consider 2.4 moles of a gas contained in a 4.0 L bulb at a constant temperature of 32°C. This bulb is connected by a valve to an evacuated 20.0 L bulb. Assume the temperature is constant. c) Given your answer to part b, what is the driving force for the process? 16.1 Chapter 16 | Slide 75 Copyright © Houghton Mifflin Company. All rights reserved. The Expansion of An Ideal Gas Into an Evacuated Bulb 16.1 Chapter 16 | Slide 76 Copyright © Houghton Mifflin Company. All rights reserved. Entropy • The driving force for a spontaneous process is an increase in the entropy of the universe. • A measure of molecular randomness or disorder. 16.1 Chapter 16 | Slide 77 Copyright © Houghton Mifflin Company. All rights reserved. Entropy • Thermodynamic function that describes the number of arrangements that are available to a system existing in a given state. • Nature spontaneously proceeds toward the states that have the highest probabilities of existing. 16.1 Chapter 16 | Slide 78 Copyright © Houghton Mifflin Company. All rights reserved. The Microstates That Give a Particular Arrangement (State) 16.1 Chapter 16 | Slide 79 Copyright © Houghton Mifflin Company. All rights reserved. Positional Entropy • A gas expands into a vacuum because the expanded state has the highest positional probability of states available to the system. • Therefore: Ssolid < Sliquid << Sgas 16.1 Chapter 16 | Slide 80 Copyright © Houghton Mifflin Company. All rights reserved. Concept Check • Predict the sign of S for each of the following, and explain: a) The evaporation of alcohol b) The freezing of water c) Compressing an ideal gas at constant temperature d) Heating an ideal gas at constant pressure e) Dissolving NaCl in water 16.1 Chapter 16 | Slide 81 Copyright © Houghton Mifflin Company. All rights reserved. Second Law of Thermodynamics • The entropy of the universe is increasing. • The total energy of the universe is constant, but the entropy is increasing. Suniverse = ΔSsystem + ΔSsurroundings 16.2 Chapter 16 | Slide 82 Copyright © Houghton Mifflin Company. All rights reserved. Concept Check • For the process A(l) A(s), which direction involves an increase in energy randomness? Positional randomness? Explain your answer. • As temperature increases/decreases (answer for both), which takes precedence? Why? • At what temperature is there a balance between energy randomness and positional randomness? 16.3 Chapter 16 | Slide 83 Copyright © Houghton Mifflin Company. All rights reserved. Concept Check Describe the following as spontaneous/nonspontaneous/cannot tell, and explain. A reaction that is: a) Exothermic and becomes more positionally random b) Exothermic and becomes less positionally random c) Endothermic and becomes more positionally random d) Endothermic and becomes less positionally random Explain how temperature affects your answers. 16.3 Chapter 16 | Slide 84 Copyright © Houghton Mifflin Company. All rights reserved. Concept Check Use the ideas of energy randomness and positional randomness in your discussion of the following: a) Under what conditions is the freezing of water spontaneous? Why? b) Under what conditions is the melting of ice spontaneous? Why? c) Under what conditions is the freezing of water as likely as the melting of ice? Why? 16.3 Chapter 16 | Slide 85 Copyright © Houghton Mifflin Company. All rights reserved. ΔSsurr • The sign of ΔSsurr depends on the direction of the heat flow. • The magnitude of ΔSsurr depends on the temperature. ΔSsurr = –ΔH/T 16.3 Chapter 16 | Slide 86 Copyright © Houghton Mifflin Company. All rights reserved. Interplay of Ssys and Ssurr in Determining the Sign of Suniv 16.3 Chapter 16 | Slide 87 Copyright © Houghton Mifflin Company. All rights reserved. Free Energy (G) • ΔSuniv = –ΔG/T (at constant T and P) • A process (at constant T and P) is spontaneous in the direction in which the free energy decreases. – Negative ΔG means positive ΔSuniv. 16.4 Chapter 16 | Slide 88 Copyright © Houghton Mifflin Company. All rights reserved. Free Energy (G) • ΔG = ΔH – TΔS (at constant T and P) 16.4 Chapter 16 | Slide 89 Copyright © Houghton Mifflin Company. All rights reserved. Concept Check • A liquid is vaporized at its boiling point. Predict the signs of w, q, H, S, Ssurr and G. • Explain your answers. 16.4 Chapter 16 | Slide 90 Copyright © Houghton Mifflin Company. All rights reserved. Exercise • The value of Hvaporization of substance X is 45.7 kJ/mol, and its normal boiling point is 72.5°C. • Calculate S, Ssurr, and G for the vaporization of one mole of this substance at 72.5°C and 1 atm. 16.4 Chapter 16 | Slide 91 Copyright © Houghton Mifflin Company. All rights reserved. Spontaneous Reactions 16.4 Chapter 16 | Slide 92 Copyright © Houghton Mifflin Company. All rights reserved. Effect of H and S on Spontaneity H S Result + spontaneous at all temps + + spontaneous at high temps spontaneous at low temps + not spontaneous at any temp 16.4 Chapter 16 | Slide 93 Copyright © Houghton Mifflin Company. All rights reserved. Concept Check • Gas A2 reacts with gas B2 to form gas AB at constant temperature and pressure. The bond energy of AB is much greater than that of either reactant. • Predict the signs of: H Ssurr S Suniv • Explain. 16.5 Chapter 16 | Slide 94 Copyright © Houghton Mifflin Company. All rights reserved. Third Law of Thermodynamics • The entropy of a perfect crystal at 0 K is zero. • The entropy of a substance increases with temperature. 16.5 Chapter 16 | Slide 95 Copyright © Houghton Mifflin Company. All rights reserved. Standard Entropy Values (S°) • Represent the increase in entropy that occurs when a substance is heated from 0 K to 298 K at 1 atm pressure. ΔS°reaction = ΣnpS°products – ΣnrS°reactants 16.5 Chapter 16 | Slide 96 Copyright © Houghton Mifflin Company. All rights reserved. Standard Free Energy Change (ΔG°) • The change in free energy that will occur if the reactants in their standard states are converted to the products in their standard states. ΔG° = ΔH° – TΔS° ΔG°reaction = ΣnpG°products – ΣnrG°reactants 16.6 Chapter 16 | Slide 97 Copyright © Houghton Mifflin Company. All rights reserved. Concept Check • A stable diatomic molecule spontaneously forms from its atoms. • Predict the signs of: H° S° G° • Explain. 16.6 Chapter 16 | Slide 98 Copyright © Houghton Mifflin Company. All rights reserved. Concept Check • Consider the following system at equilibrium at 25°C. PCl3(g) + Cl2(g) PCl5(g) G° = −92.50 kJ • What will happen to the ratio of partial pressure of PCl5 to partial pressure of PCl3 if the temperature is raised? • Explain. 16.6 Chapter 16 | Slide 99 Copyright © Houghton Mifflin Company. All rights reserved. Free Energy and Pressure G = G° + RT ln(P) or ΔG = ΔG° + RT ln(Q) 16.7 Chapter 16 | Slide 100 Copyright © Houghton Mifflin Company. All rights reserved. Concept Check • Sketch graphs of: 1. G vs. P 2. H vs. P 3. ln(K) vs. 1/T (for both endothermic and exothermic cases) 16.7 Chapter 16 | Slide 101 Copyright © Houghton Mifflin Company. All rights reserved. Free Energy and Equilibrium • The equilibrium point occurs at the lowest value of free energy available to the reaction system. ΔG = 0 = ΔG° + RT ln(K) ΔG° = –RT ln(K) 16.8 Chapter 16 | Slide 102 Copyright © Houghton Mifflin Company. All rights reserved. Change in Free Energy to Reach Equilibrium 16.8 Chapter 16 | Slide 103 Copyright © Houghton Mifflin Company. All rights reserved. Qualitative Relationship Between the Change in Standard Free Energy and the Equilibrium Constant for a Given Reaction 16.8 Chapter 16 | Slide 104 Copyright © Houghton Mifflin Company. All rights reserved. Free Energy and Work • Maximum possible useful work obtainable from a process at constant temperature and pressure is equal to the change in free energy. wmax = ΔG 16.9 Chapter 16 | Slide 105 Copyright © Houghton Mifflin Company. All rights reserved. Free Energy and Work • Achieving the maximum work available from a spontaneous process can occur only via a hypothetical pathway. Any real pathway wastes energy. • All real processes are irreversible. 16.9 Chapter 16 | Slide 106 Copyright © Houghton Mifflin Company. All rights reserved.