
Section 12.1 Temperature and Thermal Energy
... specific heat is C. It is measured in Joules per kg Kelvin (J / kg*K). Table 12.1 p. 318 gives a list of Common Specific Heats The Heat Gained or Lost by an object as its temperature changes depends on the mass, the change in temperature and the specific heat of the substance. Heat Transfer – is equ ...
... specific heat is C. It is measured in Joules per kg Kelvin (J / kg*K). Table 12.1 p. 318 gives a list of Common Specific Heats The Heat Gained or Lost by an object as its temperature changes depends on the mass, the change in temperature and the specific heat of the substance. Heat Transfer – is equ ...
Chapter 9: Thermodynamic Processes and Thermochemistry
... because it implies that heat is a substance that is contained in matter. Instead heat (like work) is a way in which energy is exchanged between a system and its surroundings. Ball B gains PE because work was done by ball A on B. Because work is force acting over a distance, work is required to raise ...
... because it implies that heat is a substance that is contained in matter. Instead heat (like work) is a way in which energy is exchanged between a system and its surroundings. Ball B gains PE because work was done by ball A on B. Because work is force acting over a distance, work is required to raise ...
Energy and Heat Transfer
... The heat capacity of a substance per unit mass Can be thought of a measure of the heat energy needed to heat 1 g of an object by 1ºC Different objects have different specific heat values ...
... The heat capacity of a substance per unit mass Can be thought of a measure of the heat energy needed to heat 1 g of an object by 1ºC Different objects have different specific heat values ...
Test 4
... 1) Define, identify and/or give examples of: electron configuration, Aufbau Principle, Hund’s Rule, Pauli Exclusion Principle, ground state, excited state, degenerate orbital, shielding, effective nuclear charge, valence electrons, valence shell, s, p, d, & f block, atomic radius, periodic trends of ...
... 1) Define, identify and/or give examples of: electron configuration, Aufbau Principle, Hund’s Rule, Pauli Exclusion Principle, ground state, excited state, degenerate orbital, shielding, effective nuclear charge, valence electrons, valence shell, s, p, d, & f block, atomic radius, periodic trends of ...
nuc_alchemy_talk-fgs-dec07
... • 197Pt is radioactive and ‘beta-decays’ to make 197Au (i.e., normal ‘stable’ gold’ ...
... • 197Pt is radioactive and ‘beta-decays’ to make 197Au (i.e., normal ‘stable’ gold’ ...
CHEMISTRY – UNITS 3 and 4 REVIEW PACKET Name Date
... What is the total mass of the nucleus of the atom? ____________________________________ What is the total mass of the atom (including electrons)? ____________________________ ...
... What is the total mass of the nucleus of the atom? ____________________________________ What is the total mass of the atom (including electrons)? ____________________________ ...
Presentation
... Critical temperature—the temp. above which a gas cannot be liquefied at any pressure Critical pressure—the pressure required to liquefy the gas at critical temperature ...
... Critical temperature—the temp. above which a gas cannot be liquefied at any pressure Critical pressure—the pressure required to liquefy the gas at critical temperature ...
Thermochem
... The reactants have less potential energy than do the products. Energy must be input in order to raise the particles up to the higher energy level. Energy + A + B --> AB ...
... The reactants have less potential energy than do the products. Energy must be input in order to raise the particles up to the higher energy level. Energy + A + B --> AB ...
Physics 20 Energy – Elastic Potential Energy - ND
... the spring and seeing how much it stretches. If Fg is graphed as a function of __________ _______________ the slope of the graph will be the ______________ ______________. Elastic potential energy is stored in such devices as bows, springs, bent poles and bungee cords. (Anything that is a Hooke's la ...
... the spring and seeing how much it stretches. If Fg is graphed as a function of __________ _______________ the slope of the graph will be the ______________ ______________. Elastic potential energy is stored in such devices as bows, springs, bent poles and bungee cords. (Anything that is a Hooke's la ...
[2013 question paper]
... [I] Consider a simple pendulum consisting of a bob of mass m suspended from a fixed support via a massless rigid rod of length l. It is free to oscillate (not necessarily through small angles) in a plane, under the influence of the Earth’s constant acceleration due to gravity g. 1. Write the Lagrang ...
... [I] Consider a simple pendulum consisting of a bob of mass m suspended from a fixed support via a massless rigid rod of length l. It is free to oscillate (not necessarily through small angles) in a plane, under the influence of the Earth’s constant acceleration due to gravity g. 1. Write the Lagrang ...
The Conservation of Energy
... ~ is the [finite] displacement from the beginning of the path to the end of the path. where ∆s Conservation of energy: The “law” of conservation of energy states that in a closed system, the total energy of the system does not change. If only conservative forces do work, this energy is purely in mec ...
... ~ is the [finite] displacement from the beginning of the path to the end of the path. where ∆s Conservation of energy: The “law” of conservation of energy states that in a closed system, the total energy of the system does not change. If only conservative forces do work, this energy is purely in mec ...
Heat transfer physics

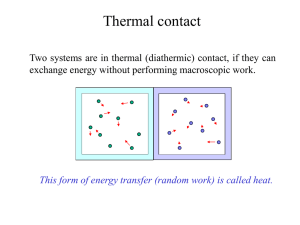
Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.

















![[2013 question paper]](http://s1.studyres.com/store/data/008881813_1-433cb609ef4aa3f6141509bf2df16e48-300x300.png)


