Unit G484: The Newtonian World
... Number of oscillations/cycles per unit time Product of 2 x frequency or 2 /period The angle, in radians between subsequent wave peaks. Acceleration is (directly) proportional to displacement (from the equilibrium position) and is always acting towards the equilibrium position. Of a gas: Collisions w ...
... Number of oscillations/cycles per unit time Product of 2 x frequency or 2 /period The angle, in radians between subsequent wave peaks. Acceleration is (directly) proportional to displacement (from the equilibrium position) and is always acting towards the equilibrium position. Of a gas: Collisions w ...
Chapter_Superconductivity
... The microscopic theory put forward by Bradeen , Cooper and Schruffier (BCS) forms the basis of quantum theory of Superconductivity. The fundamental postulate of BCS theory is that when an attractive interaction between two electrons by means of phonon exchange dominates the repulsive coulomb interac ...
... The microscopic theory put forward by Bradeen , Cooper and Schruffier (BCS) forms the basis of quantum theory of Superconductivity. The fundamental postulate of BCS theory is that when an attractive interaction between two electrons by means of phonon exchange dominates the repulsive coulomb interac ...
massachusetts institute of technology
... At the points, where E U (x) , the kinetic energy is zero. Regions where the kinetic energy is negative, are called the classically forbidden regions, which the body can never reach if subject to the laws of classical mechanics. In quantum mechanics, there is a very small probability that the body ...
... At the points, where E U (x) , the kinetic energy is zero. Regions where the kinetic energy is negative, are called the classically forbidden regions, which the body can never reach if subject to the laws of classical mechanics. In quantum mechanics, there is a very small probability that the body ...
work
... D. the unit for energy is the Joule (J), and for work too 1. 1 joule = 1 Newton x 1 meter: energy = force x distance (same as work!!) 2. so a joule is a function of the amount of force applied for a motion. Need motion to occur for energy to be used E. E = MC2: more mass = more energy F. energy is ...
... D. the unit for energy is the Joule (J), and for work too 1. 1 joule = 1 Newton x 1 meter: energy = force x distance (same as work!!) 2. so a joule is a function of the amount of force applied for a motion. Need motion to occur for energy to be used E. E = MC2: more mass = more energy F. energy is ...
File - Science With Dumars
... energies of subatomic particles. Incorporates both particle and wave behavior in terms of wave function: is proportional to the probability of finding an electron. Leads to Quantum Mechanics: we cannot pinpoint an electron in an atom but we can define the region where electrons can be in a particula ...
... energies of subatomic particles. Incorporates both particle and wave behavior in terms of wave function: is proportional to the probability of finding an electron. Leads to Quantum Mechanics: we cannot pinpoint an electron in an atom but we can define the region where electrons can be in a particula ...
lecture21
... 4. Water flows down hill where by potential energy is converted into K.E. Reverse of this process does not occur in nature. Conclusion: Processes proceed in a certain direction and not in the reverse direction. The first law places no restriction on direction. A process will not occur unless it sat ...
... 4. Water flows down hill where by potential energy is converted into K.E. Reverse of this process does not occur in nature. Conclusion: Processes proceed in a certain direction and not in the reverse direction. The first law places no restriction on direction. A process will not occur unless it sat ...
Kinetic Energy is Energy Due to Motion When the potential energy of
... steam can turn turbines. Moving molecules in a sound wave can make your eardrum vibrate. Any moving object has kinetic energy. When a solid object moves, all the molecules move in unison. The kinetic energy of such an object is often called mechanical kinetic energy. Even when molecules are not phys ...
... steam can turn turbines. Moving molecules in a sound wave can make your eardrum vibrate. Any moving object has kinetic energy. When a solid object moves, all the molecules move in unison. The kinetic energy of such an object is often called mechanical kinetic energy. Even when molecules are not phys ...
CHAPTER 2: MASS, ENERGY, AND MOMENTUM BALANCES
... Eqn. (6) is the generalized energy equation, commonly referred to as the Bernoulli equation. Examining this equation reveals that if the inlet pressure is higher than the outlet (as in the case of a turbine), the change in kinetic energy from inlet to outlet will yield negative value and the potenti ...
... Eqn. (6) is the generalized energy equation, commonly referred to as the Bernoulli equation. Examining this equation reveals that if the inlet pressure is higher than the outlet (as in the case of a turbine), the change in kinetic energy from inlet to outlet will yield negative value and the potenti ...
Lecture 4 - Purdue University
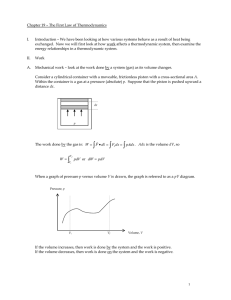
... experimental pressure volume traces (see Fig. 2.5 in text) as well as theoretical approximations to processes for defining the system behavior. Work is a path function and can not be evaluated by just knowing the end states 1 and 2. Also, in writing the above equations, the assumption that the press ...
... experimental pressure volume traces (see Fig. 2.5 in text) as well as theoretical approximations to processes for defining the system behavior. Work is a path function and can not be evaluated by just knowing the end states 1 and 2. Also, in writing the above equations, the assumption that the press ...
Text S1.
... by using the Gaussian simulation package 032. Molecular electrostatic potential for each conformer was calculated by using density functional theory (DFT) method B3LYP with the ccpVTZ basis set. The IEFPCM continuum solvent model was employed to imitate an organic solvent environment (= 4). Atomic ...
... by using the Gaussian simulation package 032. Molecular electrostatic potential for each conformer was calculated by using density functional theory (DFT) method B3LYP with the ccpVTZ basis set. The IEFPCM continuum solvent model was employed to imitate an organic solvent environment (= 4). Atomic ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.