CHAPTER TWO The First Law and Other Basic Concepts
... The first law applies to the system and surroundings, and not to the system alone. In its most basic form, the first law requires: ...
... The first law applies to the system and surroundings, and not to the system alone. In its most basic form, the first law requires: ...
Lecture 6 Free Energy
... Some commonly used units: 1 N m = 1 Joule = 107 erg = 0.239 cal. At the single molecule level, kBT is commonly used because 1 kBT ! 4 pN nm. ...
... Some commonly used units: 1 N m = 1 Joule = 107 erg = 0.239 cal. At the single molecule level, kBT is commonly used because 1 kBT ! 4 pN nm. ...
constant pressure
... as a function of temperature and volume • U(T,V), so we hold one variable (V) constant, and take the ‘partial derivative’ with respect to the other (T). ...
... as a function of temperature and volume • U(T,V), so we hold one variable (V) constant, and take the ‘partial derivative’ with respect to the other (T). ...
PS Unit 8 Study Guide Remediation ANSWERS
... 10. If mechanical energy is the sum of potential and kinetic energy of a system, calculate the GPE at its highest point and the KE right before it hits the ground, as a 200-kg model airplane lands in a grassy field after starting from 100m above the ground. ...
... 10. If mechanical energy is the sum of potential and kinetic energy of a system, calculate the GPE at its highest point and the KE right before it hits the ground, as a 200-kg model airplane lands in a grassy field after starting from 100m above the ground. ...
Document
... of substance. (J/oC·g or J/K·g) Molar heat capacity : the heat capacity is given per mole of substance. (J/oC·mol or J/K·mol) = s/ molar mass Using specific heat capacity: energy exchanged = C· m · T ex. ...
... of substance. (J/oC·g or J/K·g) Molar heat capacity : the heat capacity is given per mole of substance. (J/oC·mol or J/K·mol) = s/ molar mass Using specific heat capacity: energy exchanged = C· m · T ex. ...
CHAPTER I
... III.5. The mechanical equivalent of heat (Joule’s experiment) In the 1800s Joule spent a lot of time pondering the quantitative relationship between different forms of energy, looking to see how much is lost in converting from one form to another. As you’ll already know, when friction is present in ...
... III.5. The mechanical equivalent of heat (Joule’s experiment) In the 1800s Joule spent a lot of time pondering the quantitative relationship between different forms of energy, looking to see how much is lost in converting from one form to another. As you’ll already know, when friction is present in ...
Chapter 6 ()
... We noted in chapter 4 that the full formulation of the equations of motion required additional information to deal with the state variables density and pressure and that we were one equation short of matching unknowns and equations. In both meteorology and oceanography the variation of density and h ...
... We noted in chapter 4 that the full formulation of the equations of motion required additional information to deal with the state variables density and pressure and that we were one equation short of matching unknowns and equations. In both meteorology and oceanography the variation of density and h ...
Total view of the AFM
... characteristic and continuum X-Rays, Auger electrons, photons, and electron-hole pairs ...
... characteristic and continuum X-Rays, Auger electrons, photons, and electron-hole pairs ...
organic crystals: prediction of crystal structure from molecular structure
... the center of charges, is expanded in a series of multipoles: V(R) = A°/R +(1/R2) (Ax px+Ay py+Az pz) + (1/R3) (Az2 d z2+Axy dxy+Axz dxz+Ayz dyz+ Ax2-y2 dx2-y2) + higher terms over 1/R4 ...
... the center of charges, is expanded in a series of multipoles: V(R) = A°/R +(1/R2) (Ax px+Ay py+Az pz) + (1/R3) (Az2 d z2+Axy dxy+Axz dxz+Ayz dyz+ Ax2-y2 dx2-y2) + higher terms over 1/R4 ...
Work Energy Theorem & KE & PE
... only one force does work on the object). Gravitational, electrostatic and spring forces are conservative forces. • Friction is an example of a non-conservative force. For a round trip the frictional force generally opposes motion and only leads to a decrease in kinetic energy. ...
... only one force does work on the object). Gravitational, electrostatic and spring forces are conservative forces. • Friction is an example of a non-conservative force. For a round trip the frictional force generally opposes motion and only leads to a decrease in kinetic energy. ...
Chemistry - nyostrander.us
... 3. A specific amount of energy is emitted when excited electrons in an atom in a sample of an element return to the ground state. This emitted energy can be used to determine the (1) mass of the sample (3) identity of the element (2) volume of the sample (4) number of moles of the element 4. Accordi ...
... 3. A specific amount of energy is emitted when excited electrons in an atom in a sample of an element return to the ground state. This emitted energy can be used to determine the (1) mass of the sample (3) identity of the element (2) volume of the sample (4) number of moles of the element 4. Accordi ...
Slide 1
... 1. Stiffness and Load Vector Formulations for mechanical, heat transfer and fluid flow problems. The system equation to be solved can be written in matrix form as: [K] {D} = {q} Where [K] is traditional known as the ‘stiffness’ or ‘coefficient’ matrix (conductance matrix for heat transfer, flow-resi ...
... 1. Stiffness and Load Vector Formulations for mechanical, heat transfer and fluid flow problems. The system equation to be solved can be written in matrix form as: [K] {D} = {q} Where [K] is traditional known as the ‘stiffness’ or ‘coefficient’ matrix (conductance matrix for heat transfer, flow-resi ...
Thermodynamics
... of entropy is that it appears to depend on a path variable. But the fact that the heat transfer isn't just any type of heat transfer, but only along a special kind of path, allows it to be a state variable. Put another way, calculating changes in entropy from one state to another, we must construct ...
... of entropy is that it appears to depend on a path variable. But the fact that the heat transfer isn't just any type of heat transfer, but only along a special kind of path, allows it to be a state variable. Put another way, calculating changes in entropy from one state to another, we must construct ...
Sci Physical Science Curriculum Map Year-Long
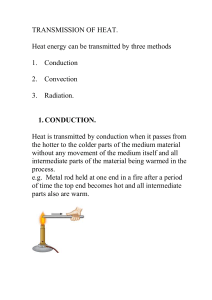
... SPS7. Students will relate transformations and flow of energy within a system. a. Identify energy transformations within a system (e.g. lighting of a match). b. Investigate molecular motion as it relates to thermal energy changes in terms of conduction, convection, and radiation. c. Determine the he ...
... SPS7. Students will relate transformations and flow of energy within a system. a. Identify energy transformations within a system (e.g. lighting of a match). b. Investigate molecular motion as it relates to thermal energy changes in terms of conduction, convection, and radiation. c. Determine the he ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.