Latent Heat of Vaporization and Speci c Heat - Physlab
... We know that molecules are always on the move as they have kinetic energy, but the question is, how is this energy shared? James Clerk Maxwell solved this problem for a large number of molecules. He said that energy is equally divided in all the directions a molecule is free to move. The average ene ...
... We know that molecules are always on the move as they have kinetic energy, but the question is, how is this energy shared? James Clerk Maxwell solved this problem for a large number of molecules. He said that energy is equally divided in all the directions a molecule is free to move. The average ene ...
Lecture 9
... Work by Constant Force Example: You pull a 30 N chest 5 meters across the floor at a constant speed by applying a force of 50 N at an angle of 30 degrees. How much work is done by the 50 N force? ...
... Work by Constant Force Example: You pull a 30 N chest 5 meters across the floor at a constant speed by applying a force of 50 N at an angle of 30 degrees. How much work is done by the 50 N force? ...
Handout 1: A More Detailed Look at Electronic Structure.
... nucleus thus quenching the orbital angular momentum and greatly reducing the magnitude of spin-orbit coupling. This is the case for the transition metals. In the lanthanide series however, the 4f orbitals are screened from the ligand electrical field by the filled 5s and 5p shells and spin-orbit cou ...
... nucleus thus quenching the orbital angular momentum and greatly reducing the magnitude of spin-orbit coupling. This is the case for the transition metals. In the lanthanide series however, the 4f orbitals are screened from the ligand electrical field by the filled 5s and 5p shells and spin-orbit cou ...
chapter12_PC
... This applies only to the reversible path, even if the system actually follows an irreversible path ...
... This applies only to the reversible path, even if the system actually follows an irreversible path ...
Document
... I guess the reason is the same one as that causing hydrogen bonding in chloroform “A hydrogen attached to carbon can also participate in hydrogen bonding when the carbon atom is bound to electronegative atoms, as is the case in chloroform, CHCl3” (from wikipedia ...
... I guess the reason is the same one as that causing hydrogen bonding in chloroform “A hydrogen attached to carbon can also participate in hydrogen bonding when the carbon atom is bound to electronegative atoms, as is the case in chloroform, CHCl3” (from wikipedia ...
File
... 8. State Stefan’s law of heat radiation. The total radiant energy emitted per second from unit area of the surface of a black body is directly proportional to the fourth power of its absolute temperature. Q = T4 . Where is Stefan’s Constant. 9. Draw the displacement-time curve for damped oscill ...
... 8. State Stefan’s law of heat radiation. The total radiant energy emitted per second from unit area of the surface of a black body is directly proportional to the fourth power of its absolute temperature. Q = T4 . Where is Stefan’s Constant. 9. Draw the displacement-time curve for damped oscill ...
VSPER, Molecular Orbitals, and Organic Molecules
... • called constructive interference: has a lower energy than the states of the isolated atoms i. anti-bonding orbital (indicated with a superscript asterisk) • electrons tend to spend more of their time not between the nuclei • tends to weaken the bond • called destructive interference: has a higher ...
... • called constructive interference: has a lower energy than the states of the isolated atoms i. anti-bonding orbital (indicated with a superscript asterisk) • electrons tend to spend more of their time not between the nuclei • tends to weaken the bond • called destructive interference: has a higher ...
Chapter 12
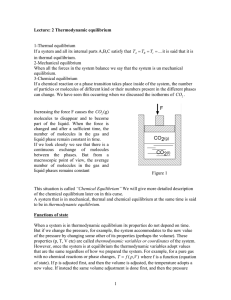
... energy, Qr, transferred along the reversible path divided by the absolute temperature, T, of the system in this ...
... energy, Qr, transferred along the reversible path divided by the absolute temperature, T, of the system in this ...
Chapter 12
... energy, Qr, transferred along the reversible path divided by the absolute temperature, T, of the system in this ...
... energy, Qr, transferred along the reversible path divided by the absolute temperature, T, of the system in this ...
ElectronicStructureSurfaces.pdf
... Screening: if we take a positive charge and "bury" it in an electron gas, the electrons in the locality of the charge will rearrange to compensate – to screen out – the positive charge. The Coulomb potential associated with the isolated charge will thus differ from that for a charge in vacuum. The ...
... Screening: if we take a positive charge and "bury" it in an electron gas, the electrons in the locality of the charge will rearrange to compensate – to screen out – the positive charge. The Coulomb potential associated with the isolated charge will thus differ from that for a charge in vacuum. The ...
Notes
... What is the energy required to vaporize water at 100oC??? when one mole of water is vaporized at 100oC the work is w = p V = RT = 1.987 cal K-1 mole-1 x 373.15K w= 741.4 cal mole-1 The energy or heat required to vaporize water at 100oC requires energy to separate the liquid molecules; that is 5 ...
... What is the energy required to vaporize water at 100oC??? when one mole of water is vaporized at 100oC the work is w = p V = RT = 1.987 cal K-1 mole-1 x 373.15K w= 741.4 cal mole-1 The energy or heat required to vaporize water at 100oC requires energy to separate the liquid molecules; that is 5 ...
Work, Energy and Momentum Notes
... Zeroth Law of Thermodynamics: Thermal energy will be transferred from a _____________ object to a _____________ object until thermal equilibrium is reached. ...
... Zeroth Law of Thermodynamics: Thermal energy will be transferred from a _____________ object to a _____________ object until thermal equilibrium is reached. ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.