Energy and Metabolism
... • NAD+ gains H atom (is reduced) to form NADH • NADH stores large amounts of energy • This energy is transferred in a series of reactions to other molecules that will eventually form ATP • NADP+ is chemically similar • Reduced to form NADPH, but this is not involved in ATP formation ...
... • NAD+ gains H atom (is reduced) to form NADH • NADH stores large amounts of energy • This energy is transferred in a series of reactions to other molecules that will eventually form ATP • NADP+ is chemically similar • Reduced to form NADPH, but this is not involved in ATP formation ...
Document
... G the Doppler effect wave of the correct frequency. Since the forks H resonance are identical, the second J standing waves one receives the correct frequency to begin vibrating. ...
... G the Doppler effect wave of the correct frequency. Since the forks H resonance are identical, the second J standing waves one receives the correct frequency to begin vibrating. ...
Day 58 - Tahoma
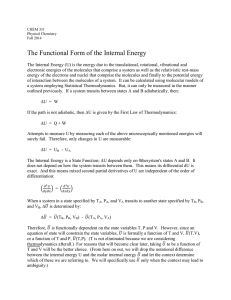
... Reversible process: The system changes so that the system and surroundings can be returned to the original state by exactly reversing the process. This maximizes work done by a system on the surroundings. ...
... Reversible process: The system changes so that the system and surroundings can be returned to the original state by exactly reversing the process. This maximizes work done by a system on the surroundings. ...
Work, Energy & Power
... more specifically, it is the ability to do work, or cause change, a transfer of energy • Every unit we have or will discuss involves energy, energy is required to produce a force, torque, velocity, light, sound, etc. Remember Momentum, an object has energy based on its movement. • Energy cannot be c ...
... more specifically, it is the ability to do work, or cause change, a transfer of energy • Every unit we have or will discuss involves energy, energy is required to produce a force, torque, velocity, light, sound, etc. Remember Momentum, an object has energy based on its movement. • Energy cannot be c ...
Chapter 7 Energy of a system Conceptual question Q7.1 Can kinetic
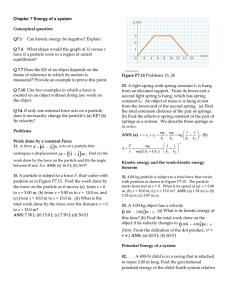
... particle within a system due to its intteaction with the rest of the system. The equation Fx = (2x + 4) N describes the force, where x is in meters. As the particle moves along the x axis from x = 1.00 m to x = 5.00 m, calculate (a) the work done by this force, (b) the change in the potential energy ...
... particle within a system due to its intteaction with the rest of the system. The equation Fx = (2x + 4) N describes the force, where x is in meters. As the particle moves along the x axis from x = 1.00 m to x = 5.00 m, calculate (a) the work done by this force, (b) the change in the potential energy ...
Chapter 7 Energy of a system Conceptual question Q7.1 Can kinetic
... 19. If it takes 4.00 J of work to stretch a Hooke'slaw spring 10.0 cm from its unstressed length, determine the extra work required to stretch it an additional 10.0 cm. ANS: 12.0 J. 3.Batman, whose mass is 80.0 kg, is dangling on the free end of a 12.0-m rope, the other end of which is fixed to a tr ...
... 19. If it takes 4.00 J of work to stretch a Hooke'slaw spring 10.0 cm from its unstressed length, determine the extra work required to stretch it an additional 10.0 cm. ANS: 12.0 J. 3.Batman, whose mass is 80.0 kg, is dangling on the free end of a 12.0-m rope, the other end of which is fixed to a tr ...
C 3 HAPTER
... focused onto the sample surface. The beam is deflected in a scanning pattern over the sample, and interaction between the electron beam with atoms at or near the sample surface generates a variety of signals including: secondary electrons, backscattered electrons, characteristic X-rays, etc. (Philip ...
... focused onto the sample surface. The beam is deflected in a scanning pattern over the sample, and interaction between the electron beam with atoms at or near the sample surface generates a variety of signals including: secondary electrons, backscattered electrons, characteristic X-rays, etc. (Philip ...
Unit 1B1 - Uddingston Grammar School
... Atoms P and Q have the same number of protons Atoms Q and R have the same number of electrons Atoms P and S have the same number of neutrons Atoms R and S are isotopes of each other Atoms S and T have different chemical properties. ...
... Atoms P and Q have the same number of protons Atoms Q and R have the same number of electrons Atoms P and S have the same number of neutrons Atoms R and S are isotopes of each other Atoms S and T have different chemical properties. ...
PYP001-121 Major-I Solution. In all the questions, choice
... Q1. Which of the following statements is FALSE? A) Smoke is a compound. B) A pure substance can be either an element or compound. C) A fruit salad is a heterogeneous mixture. D) Every type of atom has a different number of protons. E) The change of state from a gas to a liquid is called condensation ...
... Q1. Which of the following statements is FALSE? A) Smoke is a compound. B) A pure substance can be either an element or compound. C) A fruit salad is a heterogeneous mixture. D) Every type of atom has a different number of protons. E) The change of state from a gas to a liquid is called condensation ...
Chapter 10
... • Classify each process as exothermic or endothermic. Explain why. (The system is underlined.) Exo ...
... • Classify each process as exothermic or endothermic. Explain why. (The system is underlined.) Exo ...
The Ideal Gas Law and the Kinetic Theory of Gasses
... the system as an increase in internal energy. Heating a gas in a constant volume container is an example. Isobaric Process: An isobaric process is a constant pressure process. In general none of the three quantities are zero but we can calculate the work nonetheless: W = pV. Boiling water at a cons ...
... the system as an increase in internal energy. Heating a gas in a constant volume container is an example. Isobaric Process: An isobaric process is a constant pressure process. In general none of the three quantities are zero but we can calculate the work nonetheless: W = pV. Boiling water at a cons ...
Simple Harmonic Motion
... •Energy is conserved as the elastic potential energy in a spring can be converted into kinetic energy. Once again the displacement of a spring is symbolic of the amplitude of a wave •Since BOTH algebraic expressions have the ratio of the Amplitude to the velocity we can set them equal to each other. ...
... •Energy is conserved as the elastic potential energy in a spring can be converted into kinetic energy. Once again the displacement of a spring is symbolic of the amplitude of a wave •Since BOTH algebraic expressions have the ratio of the Amplitude to the velocity we can set them equal to each other. ...
Notes in pdf format
... can be found form W = PΔV. Once the work is known, the first law of thermodynamics, ΔU = Q - W, can be used to find the change in internal energy, provided a value fot the heat Q can be found. The heat needed to raise the temperature can be obtained from Q = cmΔT, where c = 4186 J/(kg C) is the spec ...
... can be found form W = PΔV. Once the work is known, the first law of thermodynamics, ΔU = Q - W, can be used to find the change in internal energy, provided a value fot the heat Q can be found. The heat needed to raise the temperature can be obtained from Q = cmΔT, where c = 4186 J/(kg C) is the spec ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.