P4 revision
... Friction between solid surfaces which are gripping. Eg. Earths crust. Friction between solid surfaces which are sliding past each other. Eg. Pieces of a car engine. Friction or drag from from fluids(liquids or gases) ...
... Friction between solid surfaces which are gripping. Eg. Earths crust. Friction between solid surfaces which are sliding past each other. Eg. Pieces of a car engine. Friction or drag from from fluids(liquids or gases) ...
As a system asymptotically approaches absolute zero of
... and the entropy of the system asymptotically approaches a minimum value; also stated as: "the entropy of all systems and of all states of a system is smallest at absolute zero" or equivalently "it is impossible to reach the absolute zero of temperature by any finite number of processes." Absolute ze ...
... and the entropy of the system asymptotically approaches a minimum value; also stated as: "the entropy of all systems and of all states of a system is smallest at absolute zero" or equivalently "it is impossible to reach the absolute zero of temperature by any finite number of processes." Absolute ze ...
Thermodynamics
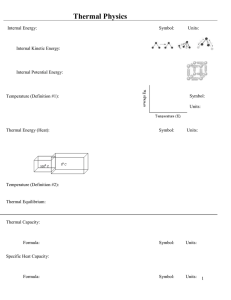
... In thermodynamics, thermodynamics the internal energy of a thermodynamic system, or a body with well-defined boundaries, denoted by U, or sometimes E, is the total of the kinetic energy due to the motion of molecules (translational, rotational, vibrational) and the potential energy associated with t ...
... In thermodynamics, thermodynamics the internal energy of a thermodynamic system, or a body with well-defined boundaries, denoted by U, or sometimes E, is the total of the kinetic energy due to the motion of molecules (translational, rotational, vibrational) and the potential energy associated with t ...
The Laws of Thermodinamics
... An isolated system does not interact with its surroundings No energy transfer takes place and no work is done Therefore, the internal energy of the isolated system remains constant ...
... An isolated system does not interact with its surroundings No energy transfer takes place and no work is done Therefore, the internal energy of the isolated system remains constant ...
7-1 The Law of Conservation of Energy
... Wnc is the work done by non-conservative forces (such as by the force of friction). The conservation of energy equation is very flexible. So far, we have discussed one form of kinetic energy, the translational kinetic energy given by . When we get to Chapter 11, we will be able to build rotational k ...
... Wnc is the work done by non-conservative forces (such as by the force of friction). The conservation of energy equation is very flexible. So far, we have discussed one form of kinetic energy, the translational kinetic energy given by . When we get to Chapter 11, we will be able to build rotational k ...
Quiz_MATH.rtf
... D) decreases at high temperature, increases at low E) stays the same 13. (C) Two monatomic ideal gases are in thermal equilibrium with each other. Gas A is composed of molecules with mass m while gas B is composed of molecules with mass 4m. The ratio of the average molecular kinetic energy KA/KB is ...
... D) decreases at high temperature, increases at low E) stays the same 13. (C) Two monatomic ideal gases are in thermal equilibrium with each other. Gas A is composed of molecules with mass m while gas B is composed of molecules with mass 4m. The ratio of the average molecular kinetic energy KA/KB is ...
Introduction in energy systems - Faculty of Mechanical Engineering
... surrounding objects (Kelvin statement). Third law of thermodynamics The system is not possible cooled, in a finite number of steps, to absolute zero. ...
... surrounding objects (Kelvin statement). Third law of thermodynamics The system is not possible cooled, in a finite number of steps, to absolute zero. ...
Forces, Work and Energy
... There are many other examples of forces which allow potential energy to be stored-the Hooke’s law force associated with deflection of a spring is another familiar example. For forces of this type, in the absence of applied external forces the sum of the kinetic and potential energies is conserved KE ...
... There are many other examples of forces which allow potential energy to be stored-the Hooke’s law force associated with deflection of a spring is another familiar example. For forces of this type, in the absence of applied external forces the sum of the kinetic and potential energies is conserved KE ...
Document
... • Temperature tells us whether or not two systems will change when brought into thermal contact with one another. • A thermometer is just a very small system with only one state variable. ...
... • Temperature tells us whether or not two systems will change when brought into thermal contact with one another. • A thermometer is just a very small system with only one state variable. ...
CHAPTER 16
... The electrons are called the majority carries in n-type material ( the n stand for the negative charge on an electron) Holes which are not produced by the addition of the pentavalent impurity atoms are called minority carries ...
... The electrons are called the majority carries in n-type material ( the n stand for the negative charge on an electron) Holes which are not produced by the addition of the pentavalent impurity atoms are called minority carries ...
unit 9: thermal physics
... the intermolecular forces and move about so that in time the entire solid becomes a liquid. As heating continues, the temperature of the liquid increases due to an increase in the vibrational, translational and rotational kinetic energy of the molecules. At the boiling point, a temperature is reache ...
... the intermolecular forces and move about so that in time the entire solid becomes a liquid. As heating continues, the temperature of the liquid increases due to an increase in the vibrational, translational and rotational kinetic energy of the molecules. At the boiling point, a temperature is reache ...
First Law of Thermodynamics
... The contribution to heat capacity CVm for a gas at a temperature of T not much lower than 300 K is R/2 for each translation and rotational degree of freedom, where R is the ideal gas constant. Each vibrational degree of freedom for which the relation E/kT < 0.1 (is active) contributes R to CVm. If ...
... The contribution to heat capacity CVm for a gas at a temperature of T not much lower than 300 K is R/2 for each translation and rotational degree of freedom, where R is the ideal gas constant. Each vibrational degree of freedom for which the relation E/kT < 0.1 (is active) contributes R to CVm. If ...
MAE 320 – Thermodynamics
... Pbottom = Patm + PH20 = Patm + ρ g z , where ρ = 1000 kg/m 3 , g = 9.81 N / kg , and z is the (to ...
... Pbottom = Patm + PH20 = Patm + ρ g z , where ρ = 1000 kg/m 3 , g = 9.81 N / kg , and z is the (to ...
Chem161 Chapter 6
... • Heat of reaction: the amount of heat absorbed or released in a chemical reaction • Calorimeter: an apparatus used to measure temperature changes in materials surrounding a reaction that result from a chemical reaction • From the temperature changes we can calculate the heat of the reaction, q ...
... • Heat of reaction: the amount of heat absorbed or released in a chemical reaction • Calorimeter: an apparatus used to measure temperature changes in materials surrounding a reaction that result from a chemical reaction • From the temperature changes we can calculate the heat of the reaction, q ...
LAW: The first law of thermodynamics states that the total energy in
... The heat capacity at constant volume is defined as: DEFINITION: The heat capacity at constant pressure is defined as: ...
... The heat capacity at constant volume is defined as: DEFINITION: The heat capacity at constant pressure is defined as: ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.