Potential energy
... • The ax has the most __________ energy at its highest point, just before it starts to fall. As the ax starts to fall, potential energy changes to ____________ energy. The ___________ energy of the ax is greatest just before it strikes the wood when it is moving at its ___________. The instant the a ...
... • The ax has the most __________ energy at its highest point, just before it starts to fall. As the ax starts to fall, potential energy changes to ____________ energy. The ___________ energy of the ax is greatest just before it strikes the wood when it is moving at its ___________. The instant the a ...
Physical Science Notes ppt.SBP1
... Light (radiant) energy is electromagnetic energy that travels in transverse waves. Electrical energy is the movement of electrical charges. Sound is the movement of energy through substances in longitudinal waves ...
... Light (radiant) energy is electromagnetic energy that travels in transverse waves. Electrical energy is the movement of electrical charges. Sound is the movement of energy through substances in longitudinal waves ...
Conservative forces and the potential energy function
... or Wnc = E mech2 # E mech1 = "E mech . Therefore, the work done by a non-conservative force is equal to the change in mechanical energy. ...
... or Wnc = E mech2 # E mech1 = "E mech . Therefore, the work done by a non-conservative force is equal to the change in mechanical energy. ...
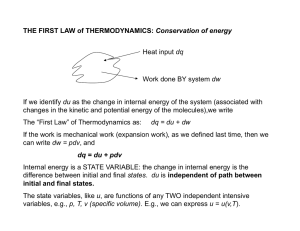
Thermodynamics
... Everything else is defined as heat. Heat is the defined as the transfer of energy to a body that does not involve work or those transfers of energy that occur only because of a difference in temperature ...
... Everything else is defined as heat. Heat is the defined as the transfer of energy to a body that does not involve work or those transfers of energy that occur only because of a difference in temperature ...
Thermodynamics of ideal gases
... air, using essentially the ideal gas law with constant temperature. His result did not agree with experiment, because normal sound waves oscillate so rapidly that compression and expansion are essentially isentropic processes. The speed of sound is c= ...
... air, using essentially the ideal gas law with constant temperature. His result did not agree with experiment, because normal sound waves oscillate so rapidly that compression and expansion are essentially isentropic processes. The speed of sound is c= ...
The Equipartition Theorem
... There is an important principle lurking behind many of the calculations we have carried out so far – the principle of the Equipartition of Energy. This is a very powerful concept in classical statistical physics and has general applicability for systems in thermal equilibrium. We will find that the ...
... There is an important principle lurking behind many of the calculations we have carried out so far – the principle of the Equipartition of Energy. This is a very powerful concept in classical statistical physics and has general applicability for systems in thermal equilibrium. We will find that the ...
Review Sheet for Benchmark Exam
... When you do an experiment do you want to control the independent variable, the dependent variable or both? ...
... When you do an experiment do you want to control the independent variable, the dependent variable or both? ...
2. Electrons in Metals - Particle Physics
... µ = EF , which is defined at 0 K. We shall take sodium metal as an example, and calculate kB T and EF for this metal. In sodium, each atom has one valence electron. This electron is mobile and forms the electron gas that we are talking about. In order to calculate the Fermi energy, we need the numbe ...
... µ = EF , which is defined at 0 K. We shall take sodium metal as an example, and calculate kB T and EF for this metal. In sodium, each atom has one valence electron. This electron is mobile and forms the electron gas that we are talking about. In order to calculate the Fermi energy, we need the numbe ...
Chapter 12
... An ideal gas absorbs 5000 J of energy while doing 2000 J of work on the environment during a constant pressure process. (a) Compute the change in internal energy of the gas. (b) If the internal energy drops by 4500 J and 2000 J is expelled from the system, find the change in volume assuming a consta ...
... An ideal gas absorbs 5000 J of energy while doing 2000 J of work on the environment during a constant pressure process. (a) Compute the change in internal energy of the gas. (b) If the internal energy drops by 4500 J and 2000 J is expelled from the system, find the change in volume assuming a consta ...
幻灯片 1
... a) What is the net work of this cycle? b) Indicate the path in the cycle along which heat is rejected. How do you know?(A sentence or two is required, perhaps bolstered by an ...
... a) What is the net work of this cycle? b) Indicate the path in the cycle along which heat is rejected. How do you know?(A sentence or two is required, perhaps bolstered by an ...
HNRS 227 Lecture #2 Chapters 2 and 3
... and the collisions between individual molecules become more violent. Since the molecules are ...
... and the collisions between individual molecules become more violent. Since the molecules are ...
HNRS 227 Lecture #2 Chapters 2 and 3
... When a satellite is a distance R from the center of Earth, the force due to gravity on the satellite is F. What is the force due to gravity on the satellite when its distance from the center of the Earth is 2R? A. 9 F B. 4 F C. F / 4 D. F / 9 ...
... When a satellite is a distance R from the center of Earth, the force due to gravity on the satellite is F. What is the force due to gravity on the satellite when its distance from the center of the Earth is 2R? A. 9 F B. 4 F C. F / 4 D. F / 9 ...
revision - metc instructors collab site
... States that the total energy stored in a body, or system, is termed enthalpy (H) Defines total stored energy the sum of internal energy and the product of pressure(P) and volume (V), i.e. H = U + PV Defines potential energy as energy stored in the molecules by virtue of their vertical position above ...
... States that the total energy stored in a body, or system, is termed enthalpy (H) Defines total stored energy the sum of internal energy and the product of pressure(P) and volume (V), i.e. H = U + PV Defines potential energy as energy stored in the molecules by virtue of their vertical position above ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.