Zagazig University
... Use this time for proton to calculate distance of travelling where apply for proton where ...
... Use this time for proton to calculate distance of travelling where apply for proton where ...
Chapter 17 - Richsingiser.com
... • This equation is used to calculate the enthalpy of reaction from heats measured using constant-volume calorimetry. ...
... • This equation is used to calculate the enthalpy of reaction from heats measured using constant-volume calorimetry. ...
NkT PV = nRT PV = Pa pressure P = m volume V = moles n particles
... Given the typically large numbers of atoms or molecules within a small volume of gas, quantitative descriptions are usually in terms of macroscopic parameters: temperature, volume, and pressure versus a microscopic treatment of individual atom dynamics. An equation of state interrelates these macros ...
... Given the typically large numbers of atoms or molecules within a small volume of gas, quantitative descriptions are usually in terms of macroscopic parameters: temperature, volume, and pressure versus a microscopic treatment of individual atom dynamics. An equation of state interrelates these macros ...
Electrical properties of solids
... Fig. 2. Schematic diagram: energy bands replace energy levels for outer electrons in an assembly of atoms close together. ...
... Fig. 2. Schematic diagram: energy bands replace energy levels for outer electrons in an assembly of atoms close together. ...
Exam Review - Manistique Area Schools
... Chapter 3 Seaweed is not a substance because ______. A. It is salty B. It is a liquid C. Its composition may be different from sample to sample D. It has hydrogen as part of its composition. ...
... Chapter 3 Seaweed is not a substance because ______. A. It is salty B. It is a liquid C. Its composition may be different from sample to sample D. It has hydrogen as part of its composition. ...
Ionic crystals
... Positive ion cores be kept apart to minimize the Coulomb repulsion Valence electrons be kept apart to minimize the Coulomb repulsion Valence electrons be kept close to positive ion cores to maximize the Coulomb attraction Kinetic energy not much increased, e.g. localizing an electron in a region x; ...
... Positive ion cores be kept apart to minimize the Coulomb repulsion Valence electrons be kept apart to minimize the Coulomb repulsion Valence electrons be kept close to positive ion cores to maximize the Coulomb attraction Kinetic energy not much increased, e.g. localizing an electron in a region x; ...
Notes
... object by one degree (Celsius or Kelvin). The units of heat capacity are joules per degree, J/°C or J/K. For pure substances the heat capacity is usually given in terms of a specified amount of the substance. The heat capacity of 1 mol of a substance is called its molar heat capacity. This is simply ...
... object by one degree (Celsius or Kelvin). The units of heat capacity are joules per degree, J/°C or J/K. For pure substances the heat capacity is usually given in terms of a specified amount of the substance. The heat capacity of 1 mol of a substance is called its molar heat capacity. This is simply ...
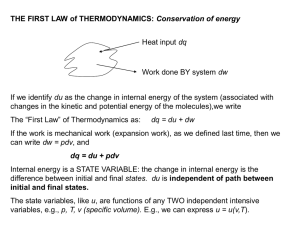
15.3 The First Law of Thermodynamics
... A heat engine is any device that uses heat to perform work. It has three essential features. 1. Heat is supplied to the engine at a relatively high temperature from a place called the hot ...
... A heat engine is any device that uses heat to perform work. It has three essential features. 1. Heat is supplied to the engine at a relatively high temperature from a place called the hot ...
Week 4 - Earth & Planetary Sciences
... radioactive decay (K,U,Th at present day) • For some bodies (e.g. Io, Europa) the principle heat source is tidal deformation (friction) • Radioactive heat production declines with time • Present-day terrestrial value ~5x10-12 W kg-1 (or ~1.5x10-8 W m-3) • Radioactive decay accounts for only about ha ...
... radioactive decay (K,U,Th at present day) • For some bodies (e.g. Io, Europa) the principle heat source is tidal deformation (friction) • Radioactive heat production declines with time • Present-day terrestrial value ~5x10-12 W kg-1 (or ~1.5x10-8 W m-3) • Radioactive decay accounts for only about ha ...
Electronic Circuit Analysis and Design Second Edition
... Nonideal Effects in Operational Amplifier Circuits Design of Integrated Circuits Feedback and Stability ...
... Nonideal Effects in Operational Amplifier Circuits Design of Integrated Circuits Feedback and Stability ...
CP-HW-ch-12
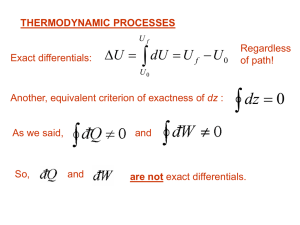
... Of the following, which is not a statement of the second law of thermodynamics? (a) No heat engine operating in a cycle can absorb energy from a reservoir and use it entirely to do work. (b) No real engine operating between two energy reservoirs can be more efficient than a Carnot engine operating b ...
... Of the following, which is not a statement of the second law of thermodynamics? (a) No heat engine operating in a cycle can absorb energy from a reservoir and use it entirely to do work. (b) No real engine operating between two energy reservoirs can be more efficient than a Carnot engine operating b ...
Sect. 2.5 - TTU Physics
... The Work done on the particle in moving it from (arbitrary) position 1 to (arbitrary) position 2 in space is defined as line integral (limits from 1 to 2): W12 ∫ Fdr • By Newton’s 2nd Law (using chain rule of differentiation): Fdr = (dp/dt)(dr/dt) dt = m(dv/dt)v dt = (½)m [d(vv)/dt] dt = (½)m ...
... The Work done on the particle in moving it from (arbitrary) position 1 to (arbitrary) position 2 in space is defined as line integral (limits from 1 to 2): W12 ∫ Fdr • By Newton’s 2nd Law (using chain rule of differentiation): Fdr = (dp/dt)(dr/dt) dt = m(dv/dt)v dt = (½)m [d(vv)/dt] dt = (½)m ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.