* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Helpful PPT on Atoms and Bonding
Survey
Document related concepts
Transcript
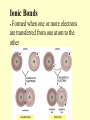
Basic chemistry Why learn it? Life depends on chemistry. If the first task of an architect is to understand building materials, then the first job of a biologist is to understand the chemistry of life. Where? Chapter 2 of your textbook What? In order: 1. The basics: Atoms and Compounds (small) 2. Properties of Water 3. Macromolecules (big) 4. Enzymes: how they get things done I anticipate a number of lab activities, at least 1 project, and 1 vocab assignment. There will also be some small homework assignments. Atoms • Atoms – The basic unit of matter. Atoms are very small, made up of subatomic particles that are even smaller than the atom. Atoms are usually neutral, meaning they have no charge. Protons • Protons Have about the same mass as a neutron. Have a positive charge Contained in nucleus in the center of the atom • Atoms have the same number of protons as electrons Neutrons • Neutrons Have about the same mass as a proton. Have no charge = neutral • Contained in nucleus in the center of the atom Electrons • Electrons Negatively charged particle Electrons are in constant motion They move around the out side of the nucleus Atoms have the same number of protons as electrons Electrons are in orbits around the nucleus The first orbit can hold 2 electrons • The second orbit can hold up to eight electrons Element • Element is a substance composed of only one type of atom more than 100 elements Elements are represented by one or two letter symbol • Sum of protons and neutrons in nucleus is called atomic mass Periodic Table • Atomic Number - Simply refers to the number of protons present in the atom. • Element Symbol - This is the symbol that chemical formulas or equations use to indicate that certain element. • Atomic Mass - The average mass of one atom of that element in amu's (Atomic Mass Units). Compounds Chemical compounds Substance formed by two or more elements Formed in definite proportions Properties of compound is different that the elements its formed by Hydrogen and oxygen are gases at room temperature, when combined they form water, a liquid, at room temperature. Bonding Chemical Bonds Atoms in a compound are held together by chemical bonds Bonds are formed by the electrons in the area around the nucleus • The electrons that are available to from bonds are called valence electrons Bonding Covalent bonds Strongest bonds Shared electrons Single covalent Bond •Two electrons are shared Double covalent bond •Four electrons are shared Ions • Ions Ions are formed by giving up or receiving an electron Ions can have a positive charge or negative charge If atom gives an electron it has a positive charge called a Cation • If atom gains an electron it has an negative charge called an Anion Ionic Bonds Formed when one or more electrons are transferred from one atom to the other Isotopes • Isotopes Have different number of neutrons They have the same number of electrons & protons • All chemical properties such as bonding remain the same Van der Waals Forces • Van der Waals Forces Not all electrons are shared equally when bonds are formed When sharing is unequal, one end of a molecule may be slightly more positive or negative than the other A slight attraction between molecules can develop because of oppositely charged regions Van der Waals forces