File - El Paso High School
... - An example of an isothermal system is a gas confined by a piston in a cylinder where the gas is not heated or cooled, but the piston is slowly moved so that the gas expands or is compressed. The temperature is maintained at a constant value by putting the system in contact with a constant-temperat ...
... - An example of an isothermal system is a gas confined by a piston in a cylinder where the gas is not heated or cooled, but the piston is slowly moved so that the gas expands or is compressed. The temperature is maintained at a constant value by putting the system in contact with a constant-temperat ...
Lecture19
... Qhot= heat that flows into engine from source at Thot Qcold= heat exhausted from engine at lower temperature, Tcold W= work done by engine ...
... Qhot= heat that flows into engine from source at Thot Qcold= heat exhausted from engine at lower temperature, Tcold W= work done by engine ...
Thermodynamics test
... 23) This person had a thermodynamic property named after him, even though he did not do any work in the invention or discovery of that property. a) Nicolas Leonard Sadi Carnot – Carnot Engine b) Anders Celsius – The Celsius Temperature Scale c) Lord Kelvin – The Kelvin Temperature Scale d) William J ...
... 23) This person had a thermodynamic property named after him, even though he did not do any work in the invention or discovery of that property. a) Nicolas Leonard Sadi Carnot – Carnot Engine b) Anders Celsius – The Celsius Temperature Scale c) Lord Kelvin – The Kelvin Temperature Scale d) William J ...
More Carnot Cycle March 4, 2010 Efficiency = W/Qin = Qin
... films (oil in water). One normally sees the colors of the rainbow. In this case, the colors observed are a result of constructive interference between light reflected from two different surfaces. There is a phase change of a light ray on reflection if the second medium has a higher index of refracti ...
... films (oil in water). One normally sees the colors of the rainbow. In this case, the colors observed are a result of constructive interference between light reflected from two different surfaces. There is a phase change of a light ray on reflection if the second medium has a higher index of refracti ...
Chapter 15
... The Second Law of Thermodynamics it as many equivalent statements Clausius statement--heat flows naturally from a hot object to a cold object, but not spontaneously (by itself without input of some kind of work) from cold to hot the study of heat engines (any device that changes thermal energy into ...
... The Second Law of Thermodynamics it as many equivalent statements Clausius statement--heat flows naturally from a hot object to a cold object, but not spontaneously (by itself without input of some kind of work) from cold to hot the study of heat engines (any device that changes thermal energy into ...
Relationships Between Heat and Work
... • Isothermal process – a thermodynamic process that takes place at constant temperature and in which the internal energy of a system remains unchanged – Similar to a balloon expanding as the pressure drops before a storm hits • The balloon expands to keep pressure equal with the environment ...
... • Isothermal process – a thermodynamic process that takes place at constant temperature and in which the internal energy of a system remains unchanged – Similar to a balloon expanding as the pressure drops before a storm hits • The balloon expands to keep pressure equal with the environment ...
Chapter 12
... The curve on the diagram is called the path taken between the initial and final states The work done depends on the particular path ...
... The curve on the diagram is called the path taken between the initial and final states The work done depends on the particular path ...
thermochemistry - Pace University Webspace
... • Exothermic- this process involves the release of energy during a chemical reaction. • Endothermic- this process involves the intake of energy during a chemical reaction. ...
... • Exothermic- this process involves the release of energy during a chemical reaction. • Endothermic- this process involves the intake of energy during a chemical reaction. ...
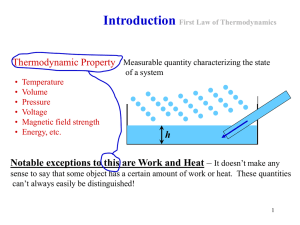
Chapter2 The First Law of Thermodynamics
... 3-1 Introduction To The First Law of Thermodynamics 3-1-1 Conservation of energy principle Energy can be neither created nor destroyed;it can only change forms 3-1-2 The First Law of Thermodynamics Neither heat nor work can be destroyed;they can only change from one to another, that is: ...
... 3-1 Introduction To The First Law of Thermodynamics 3-1-1 Conservation of energy principle Energy can be neither created nor destroyed;it can only change forms 3-1-2 The First Law of Thermodynamics Neither heat nor work can be destroyed;they can only change from one to another, that is: ...
Fuel Combustion
... pressure, the advantage of which is that more time is available for the fuel to completely combust. Because of lagging characteristics of fuel this cycle is invariably used for diesel and hot spot ignition engines. ...
... pressure, the advantage of which is that more time is available for the fuel to completely combust. Because of lagging characteristics of fuel this cycle is invariably used for diesel and hot spot ignition engines. ...