ch 6 ppt - Madison County Schools
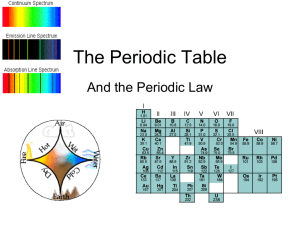
... across a period, and decrease as you move down a group. • The octet rule states that atoms gain, lose, or share electrons to acquire a full set of eight valence electrons. • Electronegativity generally increases from left to right across a period, and decreases as you move down a group. ...
... across a period, and decrease as you move down a group. • The octet rule states that atoms gain, lose, or share electrons to acquire a full set of eight valence electrons. • Electronegativity generally increases from left to right across a period, and decreases as you move down a group. ...
Chapter 6 PP
... across a period, and decrease as you move down a group. • The octet rule states that atoms gain, lose, or share electrons to acquire a full set of eight valence electrons. • Electronegativity generally increases from left to right across a period, and decreases as you move down a group. ...
... across a period, and decrease as you move down a group. • The octet rule states that atoms gain, lose, or share electrons to acquire a full set of eight valence electrons. • Electronegativity generally increases from left to right across a period, and decreases as you move down a group. ...
D. - Telluride Middle/High School
... across a period, and decrease as you move down a group. • The octet rule states that atoms gain, lose, or share electrons to acquire a full set of eight valence electrons. • Electronegativity generally increases from left to right across a period, and decreases as you move down a group. ...
... across a period, and decrease as you move down a group. • The octet rule states that atoms gain, lose, or share electrons to acquire a full set of eight valence electrons. • Electronegativity generally increases from left to right across a period, and decreases as you move down a group. ...
Document
... across a period, and decrease as you move down a group. • The octet rule states that atoms gain, lose, or share electrons to acquire a full set of eight valence electrons. • Electronegativity generally increases from left to right across a period, and decreases as you move down a group. ...
... across a period, and decrease as you move down a group. • The octet rule states that atoms gain, lose, or share electrons to acquire a full set of eight valence electrons. • Electronegativity generally increases from left to right across a period, and decreases as you move down a group. ...
2015 VCE Chemistry Unit 1 -Miss Fitzsimmons
... of the electron at the same time, which means you can't know the exact location of the electron. This principle proves error in Bohr's model because of the uncertainty of the location of an electron. ...
... of the electron at the same time, which means you can't know the exact location of the electron. This principle proves error in Bohr's model because of the uncertainty of the location of an electron. ...
CMC Chapter 5
... Section 5.2 Quantum Theory and the Atom • Compare the Bohr and quantum mechanical models of the atom. • Explain the impact of de Broglie's wave article duality and the Heisenberg uncertainty principle on the current view of electrons in atoms. • Identify the relationships among a hydrogen atom's en ...
... Section 5.2 Quantum Theory and the Atom • Compare the Bohr and quantum mechanical models of the atom. • Explain the impact of de Broglie's wave article duality and the Heisenberg uncertainty principle on the current view of electrons in atoms. • Identify the relationships among a hydrogen atom's en ...
Interactive Notebook 2 for 2011-2012
... unique properties of an element. This number of protons is called the element’s atomic number. Elements are arranged on the periodic table in order of increasing atomic number. Historically, elements were ordered by atomic mass, but now scientists know that this order would lead to misplaced element ...
... unique properties of an element. This number of protons is called the element’s atomic number. Elements are arranged on the periodic table in order of increasing atomic number. Historically, elements were ordered by atomic mass, but now scientists know that this order would lead to misplaced element ...
Atomic Structure Notepacket
... The atom is the smallest particle of an element that retains the properties of that element. Nucleus: the center of the atom; composed of neutrons and protons. Because the mass of the proton and the neutron is much larger than that of electrons, almost all the mass is located in the nucleus. Ion: a ...
... The atom is the smallest particle of an element that retains the properties of that element. Nucleus: the center of the atom; composed of neutrons and protons. Because the mass of the proton and the neutron is much larger than that of electrons, almost all the mass is located in the nucleus. Ion: a ...
chaptyer 1 - drjepmaranan
... space. For every sublevel, the value of – depends on the value of the angular momentum quantum number t and has values which range from –t passing through zero up to –t. H Hence for t = l (a p sublevel), the possible value of t are -1, 0 and +1. The number values of – which is the number of orbitals ...
... space. For every sublevel, the value of – depends on the value of the angular momentum quantum number t and has values which range from –t passing through zero up to –t. H Hence for t = l (a p sublevel), the possible value of t are -1, 0 and +1. The number values of – which is the number of orbitals ...
Atoms
... • Help make up the nucleus of the atom • Help identify the atom (could be considered an atom’s DNA) • Equal to the atomic number of the atom • Contribute to the atomic mass • Equal to the number of electrons ...
... • Help make up the nucleus of the atom • Help identify the atom (could be considered an atom’s DNA) • Equal to the atomic number of the atom • Contribute to the atomic mass • Equal to the number of electrons ...
Document
... across a period, and decrease as you move down a group. • The octet rule states that atoms gain, lose, or share electrons to acquire a full set of eight valence electrons. • Electronegativity generally increases from left to right across a period, and decreases as you move down a group. ...
... across a period, and decrease as you move down a group. • The octet rule states that atoms gain, lose, or share electrons to acquire a full set of eight valence electrons. • Electronegativity generally increases from left to right across a period, and decreases as you move down a group. ...
AP Chap 2
... • The chemical behavior of an atom is mostly determined by the valence electrons… • Atoms will bond to fill their valence electron shells. • Elements with a full valence shell are chemically inert (nonreactive) ...
... • The chemical behavior of an atom is mostly determined by the valence electrons… • Atoms will bond to fill their valence electron shells. • Elements with a full valence shell are chemically inert (nonreactive) ...
Ch 30 Nuclear Physics
... If daughter product was present when the rock formed, later analysis of the rock will result in an inaccurate parent to daughter ratio. Sometimes it may be possible to determine the amount of daughter nuclide initially present. In order to use radiometric dating techniques at all, the rocks must act ...
... If daughter product was present when the rock formed, later analysis of the rock will result in an inaccurate parent to daughter ratio. Sometimes it may be possible to determine the amount of daughter nuclide initially present. In order to use radiometric dating techniques at all, the rocks must act ...
Chapter 3: Atomic Structure
... terms of atoms. Dalton’s ATOMIC THEORY OF MATTER was based on the following postulates (see insert). 1. Each element is composed of extremely small particles called atoms. 2. All atoms of a given element are identical, but they differ from those of any other element. 3. Atoms are neither created nor ...
... terms of atoms. Dalton’s ATOMIC THEORY OF MATTER was based on the following postulates (see insert). 1. Each element is composed of extremely small particles called atoms. 2. All atoms of a given element are identical, but they differ from those of any other element. 3. Atoms are neither created nor ...
electrons
... Location of Subatomic Particles • electrons located outside nucleus • protons & neutrons located inside nucleus • protons & neutrons AKA nucleons ...
... Location of Subatomic Particles • electrons located outside nucleus • protons & neutrons located inside nucleus • protons & neutrons AKA nucleons ...
Select as many as apply.
... 3. 90Th, 91Pa, 92U 4. 26Fe, 27Co, 28Ni 5. 7N, 8O, 9F 6. 16S, 34Se, 52Te 7. I have no idea how to even begin to answer this question. ...
... 3. 90Th, 91Pa, 92U 4. 26Fe, 27Co, 28Ni 5. 7N, 8O, 9F 6. 16S, 34Se, 52Te 7. I have no idea how to even begin to answer this question. ...
AP Chemistry Summer Assignment - Belle Vernon Area School District
... 2. You need to master the formulas, charges, and names of the common ions. On the first week of the school year, you will be given a quiz on these ions. You will be asked to: • write the names of these ions when given the formula and charge • write the formula and charge when given the names I have ...
... 2. You need to master the formulas, charges, and names of the common ions. On the first week of the school year, you will be given a quiz on these ions. You will be asked to: • write the names of these ions when given the formula and charge • write the formula and charge when given the names I have ...
Investigating Atoms and Atomic Theory
... energy is quantized (has only certain values) Electrons in probability zones called “orbitals”, not orbits - location cannot be pinpointed Electrons are particles & waves at same time Electrons move around nucleus at speed of light ...
... energy is quantized (has only certain values) Electrons in probability zones called “orbitals”, not orbits - location cannot be pinpointed Electrons are particles & waves at same time Electrons move around nucleus at speed of light ...
1495/Chapter 01
... the number of electrons in the outermost, occupied shell of an atom in its lowest energy state. This shell is known as the valence shell. The electrons that occupy the valence shell of any atom are called valence electrons. The elements in the periodic table that are least reactive are the noble gas ...
... the number of electrons in the outermost, occupied shell of an atom in its lowest energy state. This shell is known as the valence shell. The electrons that occupy the valence shell of any atom are called valence electrons. The elements in the periodic table that are least reactive are the noble gas ...
Atoms - Issaquah Connect
... • Calculation: Weighted Average (info. only): – 7.5 % Lithium-6 at 6 amu – 92.5 % Lithium-7 at 7 amu ...
... • Calculation: Weighted Average (info. only): – 7.5 % Lithium-6 at 6 amu – 92.5 % Lithium-7 at 7 amu ...