Unit 2 - The Atom 1-3.key
... 1) from 2nd to 3rd shell 3) from 3rd to 1st shell 2) from 2nd to 1st shell 4) from 3rd to 2nd shell ...
... 1) from 2nd to 3rd shell 3) from 3rd to 1st shell 2) from 2nd to 1st shell 4) from 3rd to 2nd shell ...
Atoms - Issaquah Connect
... • Calculation: Weighted Average (info. only): – 7.5 % Lithium-6 at 6 amu – 92.5 % Lithium-7 at 7 amu ...
... • Calculation: Weighted Average (info. only): – 7.5 % Lithium-6 at 6 amu – 92.5 % Lithium-7 at 7 amu ...
The History of the Atom - cho
... Chemistry CPS: Project #2: GUIDELINES The History of the Atom: NAMES OF THE SCIENTISTS:__________________________________________________ _____________________________________________________________ Date assigned: ______________________________ Date due: __________________________________ P ...
... Chemistry CPS: Project #2: GUIDELINES The History of the Atom: NAMES OF THE SCIENTISTS:__________________________________________________ _____________________________________________________________ Date assigned: ______________________________ Date due: __________________________________ P ...
Q1. This question is about the first ionisation energies of some
... In a mass spectrometer, the isotopes of an element are separated. Two measurements for each isotope are recorded on the mass spectrum. State the two measurements that are recorded for each isotope. Measurement 1 .................................................................................... Mea ...
... In a mass spectrometer, the isotopes of an element are separated. Two measurements for each isotope are recorded on the mass spectrum. State the two measurements that are recorded for each isotope. Measurement 1 .................................................................................... Mea ...
Name
... What is the defined atomic mass in amu of this isotope? 21. Is the following sentence true or false? The atomic mass of an element is always a whole number of atomic mass units. 22. Circle the letter of each statement that is true about the average atomic mass of an element and the relative abundanc ...
... What is the defined atomic mass in amu of this isotope? 21. Is the following sentence true or false? The atomic mass of an element is always a whole number of atomic mass units. 22. Circle the letter of each statement that is true about the average atomic mass of an element and the relative abundanc ...
History of Atomic Theory
... • e- behaves as energy wave AND as matter particle (light also behaves as particle and wave) Einstein had predicted that energy and matter were related in his equation E = mc2 ...
... • e- behaves as energy wave AND as matter particle (light also behaves as particle and wave) Einstein had predicted that energy and matter were related in his equation E = mc2 ...
File
... • Atoms of elements with unfilled outer energy levels can form bonds. • When atoms form chemical bonds, they fill their outer energy levels with electrons and become more stable. • We will study three types of chemical bonds: ionic, covalent and metallic bonds. Mullis ...
... • Atoms of elements with unfilled outer energy levels can form bonds. • When atoms form chemical bonds, they fill their outer energy levels with electrons and become more stable. • We will study three types of chemical bonds: ionic, covalent and metallic bonds. Mullis ...
Mass Number, A
... • Protons (___) – posi2ve (+) electrical charge – mass = 1.672623 x 10-‐24 g – rela2ve mass = 1.007 atomic mass units (____) • but we can round to 1 ...
... • Protons (___) – posi2ve (+) electrical charge – mass = 1.672623 x 10-‐24 g – rela2ve mass = 1.007 atomic mass units (____) • but we can round to 1 ...
File
... 97. Which requires the most energy to raise the temperature by 1°C A. gold (c = 0.13 J/g°C) B. silver (c = 0.24 J/g°C) C. copper (c = 0.39 J/g°C) D. iron (c = 0.45 J/g°C) (58-60) You are making pasta and need to bring a pot of water to a boil. If you start with 1000 grams of water at room temperatur ...
... 97. Which requires the most energy to raise the temperature by 1°C A. gold (c = 0.13 J/g°C) B. silver (c = 0.24 J/g°C) C. copper (c = 0.39 J/g°C) D. iron (c = 0.45 J/g°C) (58-60) You are making pasta and need to bring a pot of water to a boil. If you start with 1000 grams of water at room temperatur ...
Ch 11 HW
... 2. All atoms of the same element contain the same number of __________________ 4. The _________________________ of an element is the number of protons and neutrons in the nucleus. 5. The __________________________ is an average of the masses of all naturally occurring isotopes of an element. ...
... 2. All atoms of the same element contain the same number of __________________ 4. The _________________________ of an element is the number of protons and neutrons in the nucleus. 5. The __________________________ is an average of the masses of all naturally occurring isotopes of an element. ...
Atoms
... Rutherford discovered the nucleus with gold foil & a beam of alpha particles (positive charge) ...
... Rutherford discovered the nucleus with gold foil & a beam of alpha particles (positive charge) ...
Atomic Structure-1
... mole: the SI unit used to measure the amount of a substance whose number of particles is the same as the number of atoms of carbon in exactly 12 g of carbon-12 (abbreviation, mol) ...
... mole: the SI unit used to measure the amount of a substance whose number of particles is the same as the number of atoms of carbon in exactly 12 g of carbon-12 (abbreviation, mol) ...
electrons and the structure of atoms
... The ancient Greek Democritus first proposed that matter is made up of small, indivisible particles that he called atoms. John Dalton made the first accepted theory on atoms almost 2000 years after the work of Democritus. Dalton’s atomic theory included that all atoms of an element are alike, the ato ...
... The ancient Greek Democritus first proposed that matter is made up of small, indivisible particles that he called atoms. John Dalton made the first accepted theory on atoms almost 2000 years after the work of Democritus. Dalton’s atomic theory included that all atoms of an element are alike, the ato ...
Protons and Electrons
... The energy levels are in groups that depend on their distance from the nucleus. Electrons in an energy level close to the nucleus are lower in energy than electrons in an energy level further from the nucleus. Each energy level can also contain a maximum number of electrons. For example, the first e ...
... The energy levels are in groups that depend on their distance from the nucleus. Electrons in an energy level close to the nucleus are lower in energy than electrons in an energy level further from the nucleus. Each energy level can also contain a maximum number of electrons. For example, the first e ...
Unit 23 Inside Atoms
... j) can be filled by a fixed number of electrons k) positively charged particles in the nucleus of an atom l) model where electrons occupy shells m) code name for an element n) group of atoms bonded together o) equal to the number of protons in an atom p) particle such as an electron, proton o ...
... j) can be filled by a fixed number of electrons k) positively charged particles in the nucleus of an atom l) model where electrons occupy shells m) code name for an element n) group of atoms bonded together o) equal to the number of protons in an atom p) particle such as an electron, proton o ...
Atomic Orbitals - Harding Charter Preparatory High School
... • Pauli exclusion principle – an atomic orbital may describe at most two electrons – To occupy the same orbital, the two electrons must have opposite spin represented with an up or down arrow ↑↓ ...
... • Pauli exclusion principle – an atomic orbital may describe at most two electrons – To occupy the same orbital, the two electrons must have opposite spin represented with an up or down arrow ↑↓ ...
Chemistry can be defined as the study of the composition, structure
... Therefore the first electronic shell can only contain two electrons. The second electronic shell can only contain 8 electrons…. The Atomic Number (Z) of an element is the total number of protons or electrons in the nucleus of an atom. Due to the fact that the overall charge of the atom is neutral th ...
... Therefore the first electronic shell can only contain two electrons. The second electronic shell can only contain 8 electrons…. The Atomic Number (Z) of an element is the total number of protons or electrons in the nucleus of an atom. Due to the fact that the overall charge of the atom is neutral th ...
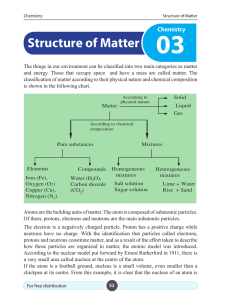
Structure of Matter - e
... Of them, protons, electrons and neutrons are the main subatomic particles. The electron is a negatively charged particle. Proton has a positive charge while neutrons have no charge. With the identification that particles called electrons, protons and neutrons constitute matter, and as a result of th ...
... Of them, protons, electrons and neutrons are the main subatomic particles. The electron is a negatively charged particle. Proton has a positive charge while neutrons have no charge. With the identification that particles called electrons, protons and neutrons constitute matter, and as a result of th ...
Section 2 Electron Configuration and the Periodic Table Chapter 5
... • Mendeleev noticed that when the elements were arranged in order of increasing atomic mass, certain similarities in their chemical properties appeared at regular intervals. • Repeating patterns are referred to as periodic. • Mendeleev created a table in which elements with similar properties were g ...
... • Mendeleev noticed that when the elements were arranged in order of increasing atomic mass, certain similarities in their chemical properties appeared at regular intervals. • Repeating patterns are referred to as periodic. • Mendeleev created a table in which elements with similar properties were g ...
S2 Chemistry - Aberdeen Grammar School
... carbon becomes part of the plant. Plants that die and are buried may turn into fossil fuels made of carbon like coal and oil over millions of years. When humans burn fossil fuels, most of the carbon quickly enters the atmosphere as carbon dioxide. Increased levels of carbon dioxide can cause changes ...
... carbon becomes part of the plant. Plants that die and are buried may turn into fossil fuels made of carbon like coal and oil over millions of years. When humans burn fossil fuels, most of the carbon quickly enters the atmosphere as carbon dioxide. Increased levels of carbon dioxide can cause changes ...
Metal found in the salt
... – Families (groups) had similar chemical and physical properties – Discovered all elements in same family had same number of valence e- -outermost electrons in highest energy level – Why? ...
... – Families (groups) had similar chemical and physical properties – Discovered all elements in same family had same number of valence e- -outermost electrons in highest energy level – Why? ...