Counting Atoms
... • Isotopes • Atoms of the same element that have different masses (different # neutrons) ...
... • Isotopes • Atoms of the same element that have different masses (different # neutrons) ...
atomic number
... The Atomic Number = # of protons in the nucleus. The Atomic Mass = # of Protons + Neutrons The number of Protons = Number of Electrons. Electrons orbit the nucleus in energy levels or electron shells. ...
... The Atomic Number = # of protons in the nucleus. The Atomic Mass = # of Protons + Neutrons The number of Protons = Number of Electrons. Electrons orbit the nucleus in energy levels or electron shells. ...
periodictrendsss - rlsciencecurriculum
... Formulas for chemical compounds can also be predicted using the periodic table. Carbon and oxygen form carbon dioxide (CO2). What formula would you predict for a compound of carbon and sulfur? Since oxygen and sulfur are in the same column, they will react with carbon in the same ratios. The best gu ...
... Formulas for chemical compounds can also be predicted using the periodic table. Carbon and oxygen form carbon dioxide (CO2). What formula would you predict for a compound of carbon and sulfur? Since oxygen and sulfur are in the same column, they will react with carbon in the same ratios. The best gu ...
Ch. 4.1 Notes - BAschools.org
... • Why: Chadwick was trying to figure out the discrepancy of atomic mass not being equal to the number of protons plus electrons. He theorized there must be a massive particle that had no charge and was therefore hard to find. ...
... • Why: Chadwick was trying to figure out the discrepancy of atomic mass not being equal to the number of protons plus electrons. He theorized there must be a massive particle that had no charge and was therefore hard to find. ...
Isotopes-Chemistry
... Same Element Different AtomIsotopes All atoms of a particular element are not exactly alike. Some elements have atoms with different masses (isotopes) ...
... Same Element Different AtomIsotopes All atoms of a particular element are not exactly alike. Some elements have atoms with different masses (isotopes) ...
Do Now
... rearranged in different combinations. Atoms of one element are never changed into atoms of another element as a result of a chemical reaction. Compound made by chemically combining atoms of elements A and B ...
... rearranged in different combinations. Atoms of one element are never changed into atoms of another element as a result of a chemical reaction. Compound made by chemically combining atoms of elements A and B ...
atomic number
... – Alpha particles rarely bounce off of thin gold foil – Leads to discovery of nucleus and proton ...
... – Alpha particles rarely bounce off of thin gold foil – Leads to discovery of nucleus and proton ...
Column A
... a. What determines the location of an electron in the electron cloud? How many energy levels are present. Electrons fill the energy levels in order (2-8-8-18) b. How many electrons can be found in the first energy level of an atom? 2 c. How many electrons can be found in the second energy level of a ...
... a. What determines the location of an electron in the electron cloud? How many energy levels are present. Electrons fill the energy levels in order (2-8-8-18) b. How many electrons can be found in the first energy level of an atom? 2 c. How many electrons can be found in the second energy level of a ...
Ch. 6 PPT
... nucleus to the valence shell KC 16: Increases by the number of Energy Levels as you move down a group, and decreases as you add protons when you move across a period ...
... nucleus to the valence shell KC 16: Increases by the number of Energy Levels as you move down a group, and decreases as you add protons when you move across a period ...
Ch. 14 notes (teacher)3
... “Electron-dot notation”: Electrons will be represented as dots located around the symbol of the element in the pattern shown below. ...
... “Electron-dot notation”: Electrons will be represented as dots located around the symbol of the element in the pattern shown below. ...
Atomic History Timeline
... • When Becquerel placed uranium salt in a desk drawer with a photographic plate, radiation from the uranium formed an image on the plate. • This proved that radiation could occur without outside energy such as the sun • When he placed metal between the uranium and photographic plates, the metal bloc ...
... • When Becquerel placed uranium salt in a desk drawer with a photographic plate, radiation from the uranium formed an image on the plate. • This proved that radiation could occur without outside energy such as the sun • When he placed metal between the uranium and photographic plates, the metal bloc ...
Name______________________________ (First and Last
... pieces like neutrons, electrons, and protons. But guess what? There are even smaller particles moving around in atoms. These super-small particles can be found inside the protons and neutrons. Scientists have many names for those pieces, but you may have heard of nucleons and quarks. Nuclear chemist ...
... pieces like neutrons, electrons, and protons. But guess what? There are even smaller particles moving around in atoms. These super-small particles can be found inside the protons and neutrons. Scientists have many names for those pieces, but you may have heard of nucleons and quarks. Nuclear chemist ...
+ The ATOM - cloudfront.net
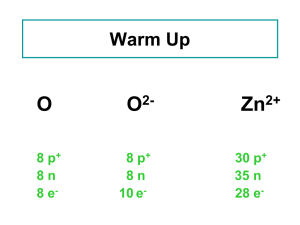
... have different numbers of electrons. • Atoms of the same element with different numbers of electrons are called ions. • When the number of protons and electrons is different, the atom becomes charged. ...
... have different numbers of electrons. • Atoms of the same element with different numbers of electrons are called ions. • When the number of protons and electrons is different, the atom becomes charged. ...
- Science
... The term matter describes all of the physical substances around us: your table, your body, a pencil, water, and so forth ...
... The term matter describes all of the physical substances around us: your table, your body, a pencil, water, and so forth ...
Distinguishing Among Atoms
... protons and neutrons in the nucleus •Example- carbon has 6 protons and 6 neutrons so the mass number is 12 •Most the mass of an atom is concentrated in the nucleus •The number of neutrons in an atom is the difference between the mass number and the atomic ...
... protons and neutrons in the nucleus •Example- carbon has 6 protons and 6 neutrons so the mass number is 12 •Most the mass of an atom is concentrated in the nucleus •The number of neutrons in an atom is the difference between the mass number and the atomic ...
File
... 6. The effective nuclear charge experienced by the outermost electron of Na is different than the effective nuclear charge experienced by the outermost electron of Ne. This difference best accounts for which of the following? A. Na has a greater density at standard conditions than Ne. B. Na has a lo ...
... 6. The effective nuclear charge experienced by the outermost electron of Na is different than the effective nuclear charge experienced by the outermost electron of Ne. This difference best accounts for which of the following? A. Na has a greater density at standard conditions than Ne. B. Na has a lo ...
Unit 1: Atoms, Molecules, and Ions
... energy and are more stable due to strong electrostatic attraction with the nucleus. The letter “n” is used to designate energy levels (orbits), with lower whole number values of n representing lower energy orbits closer to the nucleus. The key to Bohr’s Quantum Model was that electrons are restricte ...
... energy and are more stable due to strong electrostatic attraction with the nucleus. The letter “n” is used to designate energy levels (orbits), with lower whole number values of n representing lower energy orbits closer to the nucleus. The key to Bohr’s Quantum Model was that electrons are restricte ...
Homework Assignment 1 Key
... Matter is made of tiny indivisible particles called atoms o Atoms are made of smaller particles such as protons, neutrons, and electrons o Atoms can be split (nuclear fission) Atoms of the same element are identical o not true because isotopes were discovered. Isotopes of the same element contain di ...
... Matter is made of tiny indivisible particles called atoms o Atoms are made of smaller particles such as protons, neutrons, and electrons o Atoms can be split (nuclear fission) Atoms of the same element are identical o not true because isotopes were discovered. Isotopes of the same element contain di ...
Name Per ___ Reading Assignment – Chapter 4 pages 100
... mass number ___________________________________________________________________________ Nucleus ________________________________________________________________________ ____ energy levels ___________________________________________________________________________ isotopes ___________________________ ...
... mass number ___________________________________________________________________________ Nucleus ________________________________________________________________________ ____ energy levels ___________________________________________________________________________ isotopes ___________________________ ...
File
... from the elements that make them up If only covalent bonds hold atoms together, it is a molecule ...
... from the elements that make them up If only covalent bonds hold atoms together, it is a molecule ...
Ch. 3 - Atomic Structure
... Atoms of the same element with different mass numbers.(because of different #neutrons) ...
... Atoms of the same element with different mass numbers.(because of different #neutrons) ...