Ions - Van Buren Public Schools
... You have learned that not all atoms of an element are the same. Variation in the number of neutrons results in different isotopes of the element. In this activity we will explore another variation that can take place—the loss and gain of electrons. The exchange of electrons between atoms is a very c ...
... You have learned that not all atoms of an element are the same. Variation in the number of neutrons results in different isotopes of the element. In this activity we will explore another variation that can take place—the loss and gain of electrons. The exchange of electrons between atoms is a very c ...
Physical Properties
... Atomic Model – A theory proposed to explain why elements differ from each other and from nonelements. Dalton’s Atomic Model - 1808 - Matter is made up of atoms, particles to small to be seen - Each element had its own kind of atom, with its own particular mass - Compounds are made when atoms of diff ...
... Atomic Model – A theory proposed to explain why elements differ from each other and from nonelements. Dalton’s Atomic Model - 1808 - Matter is made up of atoms, particles to small to be seen - Each element had its own kind of atom, with its own particular mass - Compounds are made when atoms of diff ...
Atoms
... p-orbital: 3-dimmensional number 8; come in triplets d and f have complicated shapes. Orbitals are centered around the nucleus, and as said earlier, s, p, d and f orbitals of similar size are arranged in a shell. Larger shell engulfs all smaller ones, just like a Russian doll contains all smaller on ...
... p-orbital: 3-dimmensional number 8; come in triplets d and f have complicated shapes. Orbitals are centered around the nucleus, and as said earlier, s, p, d and f orbitals of similar size are arranged in a shell. Larger shell engulfs all smaller ones, just like a Russian doll contains all smaller on ...
9 Ions-S - 4J Blog Server
... You have learned that not all atoms of an element are the same. Variation in the number of neutrons results in different isotopes of the element. In this activity we will explore another variation that can take place—the loss and gain of electrons. The exchange of electrons between atoms is a very c ...
... You have learned that not all atoms of an element are the same. Variation in the number of neutrons results in different isotopes of the element. In this activity we will explore another variation that can take place—the loss and gain of electrons. The exchange of electrons between atoms is a very c ...
14.1 Force inside atoms
... nucleus together? !There is another force that is even stronger than the electric force. !We call it the strong nuclear force. ...
... nucleus together? !There is another force that is even stronger than the electric force. !We call it the strong nuclear force. ...
Chapter 4 Notes - Atomic Theory
... If you could collect and measure all of the exhaust from this car, you would find that mass of reactants (gas + O2) = mass of products (exhaust)! ...
... If you could collect and measure all of the exhaust from this car, you would find that mass of reactants (gas + O2) = mass of products (exhaust)! ...
20161025131513
... o what patterns did he noticeo what was his final arrangement of the periodic tableo what was missing in his tableo how did he predict undiscovered elementso was he the first to make a periodic tableo what does the placement of the elements reveal links betweeno how were his predictionsSection 2 V ...
... o what patterns did he noticeo what was his final arrangement of the periodic tableo what was missing in his tableo how did he predict undiscovered elementso was he the first to make a periodic tableo what does the placement of the elements reveal links betweeno how were his predictionsSection 2 V ...
Modern Theories of Matter
... Changes to the Dalton Model of the Atom n Discovery of electrons q William Crookes showed that as current passed through a cathode (negative electrode) in a glass vacuum tube, the tube glowed casting a shadow of the anode at the opposite end. (the glowing is called fluorescence) p the shadow showed ...
... Changes to the Dalton Model of the Atom n Discovery of electrons q William Crookes showed that as current passed through a cathode (negative electrode) in a glass vacuum tube, the tube glowed casting a shadow of the anode at the opposite end. (the glowing is called fluorescence) p the shadow showed ...
Lec: Periodic Table of Elements
... WebElements: A Periodic Table on the Web Periodic Table of Elements: Videos Interactive Periodic Table of Elements ...
... WebElements: A Periodic Table on the Web Periodic Table of Elements: Videos Interactive Periodic Table of Elements ...
ions - TeacherWeb
... You have learned that not all atoms of an element are the same. Variation in the number of neutrons results in different isotopes of the element. In this activity we will explore another variation that can take place—the loss and gain of electrons. The exchange of electrons between atoms is a very c ...
... You have learned that not all atoms of an element are the same. Variation in the number of neutrons results in different isotopes of the element. In this activity we will explore another variation that can take place—the loss and gain of electrons. The exchange of electrons between atoms is a very c ...
Chemical introduction 2016 summer
... 1. A substance composed of atoms with the same atomic number; it cannot be broken down in ordinary chemical reactions. 2.The smallest indivisible particle of matter that can have an independent existence. 3.Two or more atoms which are chemically combined to form a single species. 4. An atom that has ...
... 1. A substance composed of atoms with the same atomic number; it cannot be broken down in ordinary chemical reactions. 2.The smallest indivisible particle of matter that can have an independent existence. 3.Two or more atoms which are chemically combined to form a single species. 4. An atom that has ...
Chemistry I Honors
... c.In terms of atomic structure, explain why the first ionization energy of selenium is i. less than that of bromine (atomic number 35), and ii.greater than that of tellurium (atomic number 52). d.Selenium reacts with fluorine to form SeF4. Draw the complete Lewis electron dot structure for SeF4 and ...
... c.In terms of atomic structure, explain why the first ionization energy of selenium is i. less than that of bromine (atomic number 35), and ii.greater than that of tellurium (atomic number 52). d.Selenium reacts with fluorine to form SeF4. Draw the complete Lewis electron dot structure for SeF4 and ...
Ch 2 Notes
... Monatomic Cations Transition metals will form more than one cation Add roman numerals to name for the charge ...
... Monatomic Cations Transition metals will form more than one cation Add roman numerals to name for the charge ...
Chemistry Chapter 5 The Periodic Law
... In 1869, Dmitri Mendeleev published his first “periodic table”. – He started by placing the known elements in order using their atomic masses. – He recognized that certain properties repeated themselves “periodically”. – He then rearranged the elements so that elements with similar properties appear ...
... In 1869, Dmitri Mendeleev published his first “periodic table”. – He started by placing the known elements in order using their atomic masses. – He recognized that certain properties repeated themselves “periodically”. – He then rearranged the elements so that elements with similar properties appear ...
Chapter 6 Practice Test
... b. Hg d. Te How does atomic radius change from top to bottom in a group in the periodic table? a. It tends to decrease. c. It first increases, then decreases. b. It tends to increase. d. It first decreases, then increases. How does atomic radius change from left to right across a period in the perio ...
... b. Hg d. Te How does atomic radius change from top to bottom in a group in the periodic table? a. It tends to decrease. c. It first increases, then decreases. b. It tends to increase. d. It first decreases, then increases. How does atomic radius change from left to right across a period in the perio ...
Unit One: Atomic Theory/Configuration
... • Neutron glue that holds the nucleus together • Massive particle so adds to the atom’s mass (mass number= p + n) • Number can differ from one atom to another giving isotopes ...
... • Neutron glue that holds the nucleus together • Massive particle so adds to the atom’s mass (mass number= p + n) • Number can differ from one atom to another giving isotopes ...
Fall Exam 1
... 19. Nobel prize winner Ernest Rutherford conducted an experiment with gold foil and alpha particles, leading to the discovery that an atom’s mass is evenly distributed among its electrons. B. atoms are comprised of small particles called quarks. 20. Which of these subatomic particles has a charge of ...
... 19. Nobel prize winner Ernest Rutherford conducted an experiment with gold foil and alpha particles, leading to the discovery that an atom’s mass is evenly distributed among its electrons. B. atoms are comprised of small particles called quarks. 20. Which of these subatomic particles has a charge of ...
We cannot see an individual atom
... When certain unstable atoms spontaneously decay, emitting radiations of different kinds, and often changing into atoms of different elements, the activity takes place in the nucleus of the atom. Fission (as in a nuclear reactor or an atomic bomb) and fusion (as in the formation of elements in the Su ...
... When certain unstable atoms spontaneously decay, emitting radiations of different kinds, and often changing into atoms of different elements, the activity takes place in the nucleus of the atom. Fission (as in a nuclear reactor or an atomic bomb) and fusion (as in the formation of elements in the Su ...
Powerpoint for Scientist Contributions and the Modern Theory of
... All atoms: – Are small hard particles – Are made of a single material formed into different shapes and sizes – Are always moving, and they form different materials by joining together ...
... All atoms: – Are small hard particles – Are made of a single material formed into different shapes and sizes – Are always moving, and they form different materials by joining together ...
The Atom - Magoffin County Schools
... • Within this space, ELECTRONS move around the nucleus of the atom much like a swarm of bees around a hive. • This area is known as the ELECTRON CLOUD. ...
... • Within this space, ELECTRONS move around the nucleus of the atom much like a swarm of bees around a hive. • This area is known as the ELECTRON CLOUD. ...
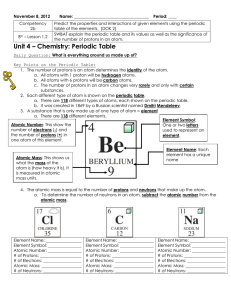
Intro to the Periodic Table
... 7. Find the element with the atomic number of 8. a. What is the element name? _________________________ b. What is the element symbol? _________________________ c. How many protons are in one atom of this element? __________________ d. How many electrons are in one atom of this element? ____________ ...
... 7. Find the element with the atomic number of 8. a. What is the element name? _________________________ b. What is the element symbol? _________________________ c. How many protons are in one atom of this element? __________________ d. How many electrons are in one atom of this element? ____________ ...
Chemistry - El Camino College
... a. In ionic reactions, atoms give or take _________ to get a full outer electron orbital b. Oppositely charged ions are strongly attracted to each other, form _______ bonds, and are called ______ or electrolytes 2. _________ Bonds are strong chemical bonds between atoms that result from the _______ ...
... a. In ionic reactions, atoms give or take _________ to get a full outer electron orbital b. Oppositely charged ions are strongly attracted to each other, form _______ bonds, and are called ______ or electrolytes 2. _________ Bonds are strong chemical bonds between atoms that result from the _______ ...