Bio_130_files/Chemistry Review
... • During each conversion, some of the energy dissipates into the environment as heat. –Heat is defined as the measure of the random motion of molecules. – the second law states that "energy systems have a tendency to increase their entropy" ...
... • During each conversion, some of the energy dissipates into the environment as heat. –Heat is defined as the measure of the random motion of molecules. – the second law states that "energy systems have a tendency to increase their entropy" ...
Atomic Structure -
... Because valence electrons are on the last shell, they are the ones that are furthest from the positive field of the protons. Valance electrons are the electrons that determine if an atom can bond with another atom. This means that the valence electrons could be attracted to the nucleus of more posit ...
... Because valence electrons are on the last shell, they are the ones that are furthest from the positive field of the protons. Valance electrons are the electrons that determine if an atom can bond with another atom. This means that the valence electrons could be attracted to the nucleus of more posit ...
Bio_130_files/Chemistry Review
... • During each conversion, some of the energy dissipates into the environment as heat. –Heat is defined as the measure of the random motion of molecules. – the second law states that "energy systems have a tendency to increase their entropy" ...
... • During each conversion, some of the energy dissipates into the environment as heat. –Heat is defined as the measure of the random motion of molecules. – the second law states that "energy systems have a tendency to increase their entropy" ...
Carbon Isotopes
... When a radioactive element decays, it may become... A different isotope of the same element A new element The original radioisotope is always referred to as the parent nuclide (the unstable nucleus), and the resulting isotope as the daughter nuclide. Many radioactive elements decay as part of a ...
... When a radioactive element decays, it may become... A different isotope of the same element A new element The original radioisotope is always referred to as the parent nuclide (the unstable nucleus), and the resulting isotope as the daughter nuclide. Many radioactive elements decay as part of a ...
The Periodic Table - Warren County Public Schools
... • Their reactivity is based on the fact that they have 7 valence electrons. Since all elements desire a full valence shell (halogens desire to get one more!) ...
... • Their reactivity is based on the fact that they have 7 valence electrons. Since all elements desire a full valence shell (halogens desire to get one more!) ...
Section 12.3
... The mass of individual atoms is so small that the numbers are difficult to work with. To make calculations easier, scientists use the atomic mass unit (amu). The atomic mass of any element is the average mass (in amu) of an atom of each element. ...
... The mass of individual atoms is so small that the numbers are difficult to work with. To make calculations easier, scientists use the atomic mass unit (amu). The atomic mass of any element is the average mass (in amu) of an atom of each element. ...
chapter 2 - Columbia University
... Mass Number, A Atoms of the same element can differ in mass number A = number of protons + number of neutrons Isotope ...
... Mass Number, A Atoms of the same element can differ in mass number A = number of protons + number of neutrons Isotope ...
CHEM 1411 CHAPTER 2
... Atomic number is taken as the basis for the arrangement of the elements, because when the elements are arranged in the increasing order of their atomic numbers, elements with similar properties repeat after a regular interval. This is called Periodic law The horizontal rows are called periods and th ...
... Atomic number is taken as the basis for the arrangement of the elements, because when the elements are arranged in the increasing order of their atomic numbers, elements with similar properties repeat after a regular interval. This is called Periodic law The horizontal rows are called periods and th ...
Notes - Zion Central Middle School
... Isotopes are atoms of the same elements that have different numbers of neutrons. o Each isotope of an element has a different mass number. ...
... Isotopes are atoms of the same elements that have different numbers of neutrons. o Each isotope of an element has a different mass number. ...
Unit 3 - The Atom
... *AIM: How can atoms of the same element be both similar and different at the same time?- Isotopes ...
... *AIM: How can atoms of the same element be both similar and different at the same time?- Isotopes ...
Lesson Guide
... 6. Conduct a flame test with the following metals: barium, strontium, sodium, copper, and lithium. a. Discuss why each metal gives off a characteristic color. b. Discuss some of the applications of this property, such as metal identification and use in logs for a fireplace. 7. Report on the scanning ...
... 6. Conduct a flame test with the following metals: barium, strontium, sodium, copper, and lithium. a. Discuss why each metal gives off a characteristic color. b. Discuss some of the applications of this property, such as metal identification and use in logs for a fireplace. 7. Report on the scanning ...
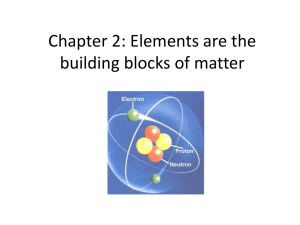
Chapter 2: Elements are the building blocks of matter
... • Organizes the elements according to their physical and chemical properties • The one we use was developed by Dmitri Mendeleev in 1867 ...
... • Organizes the elements according to their physical and chemical properties • The one we use was developed by Dmitri Mendeleev in 1867 ...
Chapter 2
... nature of combustion and like Boyle, he carefully measured the weight of reactant and product. He suggested that "Mass is neither created nor destroyed". • Lavoisier states that combustion involved Oxygen and life process used oxygen – similar to combustion. • Lavoisier published the first modern ch ...
... nature of combustion and like Boyle, he carefully measured the weight of reactant and product. He suggested that "Mass is neither created nor destroyed". • Lavoisier states that combustion involved Oxygen and life process used oxygen – similar to combustion. • Lavoisier published the first modern ch ...
Lecture Notes Part 2 - Dr. Samples` Chemistry Classes
... • The number underneath an Elemental Symbol on the Periodic Table is its atomic mass (the mass of 1 “average” atom in amu). • Is a formula mass very useful in the lab? Well, can we weigh out individual atoms or ions on a lab balance? They are too tiny to weigh or pick out individually! • Chemists we ...
... • The number underneath an Elemental Symbol on the Periodic Table is its atomic mass (the mass of 1 “average” atom in amu). • Is a formula mass very useful in the lab? Well, can we weigh out individual atoms or ions on a lab balance? They are too tiny to weigh or pick out individually! • Chemists we ...
Slide 1
... Millions of substances have been characterized by chemists. Of these, a very small number are known as elements, from which all other substances are made. An element is a substance that cannot be decomposed by any chemical reaction into simpler substances. (examples) The smallest unit of an element ...
... Millions of substances have been characterized by chemists. Of these, a very small number are known as elements, from which all other substances are made. An element is a substance that cannot be decomposed by any chemical reaction into simpler substances. (examples) The smallest unit of an element ...
Chapter 4
... 1.What did Democritus, Dalton, Thomson, Rutherford, and Bohr all have in common? 2.In Thomson’s “plum-pudding” model of the atom, the plums represent 3.An atom of gold with 79 protons, 79 electrons, and 118 neutrons would have a mass number of 4.Which subatomic particle has the least mass? 5.If an i ...
... 1.What did Democritus, Dalton, Thomson, Rutherford, and Bohr all have in common? 2.In Thomson’s “plum-pudding” model of the atom, the plums represent 3.An atom of gold with 79 protons, 79 electrons, and 118 neutrons would have a mass number of 4.Which subatomic particle has the least mass? 5.If an i ...
Per.Table.Properties. Notes
... radius than one with a small radius. It also means there is less affinity for an electron. One periodic trend, then, is that electron affinity decreases as we move down a group. For nonmetals, there is a natural tendency to want to gain electrons. Even for many metals, their electronic structure all ...
... radius than one with a small radius. It also means there is less affinity for an electron. One periodic trend, then, is that electron affinity decreases as we move down a group. For nonmetals, there is a natural tendency to want to gain electrons. Even for many metals, their electronic structure all ...
Isotopes are atoms of the same element that have different masses
... 7. An isotope of xenon has an atomic number of 54 and contains 77 neutrons. What is the mass of this isotope? 8. What is the mass number of uranium-234? 9. How many neutrons are in uranium 234? 10. Silicon is very important to the semiconductor industry. The three naturally occurring isotopes of sil ...
... 7. An isotope of xenon has an atomic number of 54 and contains 77 neutrons. What is the mass of this isotope? 8. What is the mass number of uranium-234? 9. How many neutrons are in uranium 234? 10. Silicon is very important to the semiconductor industry. The three naturally occurring isotopes of sil ...
Drawing Atomic Structures
... Atomic radius refers to the distance from ______________________________ ______________________________ ...
... Atomic radius refers to the distance from ______________________________ ______________________________ ...
The average atomic mass of an element is the sum of the
... atomic number of chlorine is 17 (it has 17 protons in its nucleus). To calculate the average mass, first convert the percentages intofractions (divide them by 100). Then, calculate the mass numbers. The chlorine isotope with 18 neutrons has an abundance of 0.7577 and a mass number of 35 amu. To calc ...
... atomic number of chlorine is 17 (it has 17 protons in its nucleus). To calculate the average mass, first convert the percentages intofractions (divide them by 100). Then, calculate the mass numbers. The chlorine isotope with 18 neutrons has an abundance of 0.7577 and a mass number of 35 amu. To calc ...
Atomic structure
... This Powerpoint is hosted on www.worldofteaching.com Please visit for 100’s more free powerpoints ...
... This Powerpoint is hosted on www.worldofteaching.com Please visit for 100’s more free powerpoints ...
Chapter 7 Periodic Properties of the Elements
... about how elements should be grouped. Periodic Properties of the Elements ...
... about how elements should be grouped. Periodic Properties of the Elements ...
1. discuss the contributions of early scie
... Noble Gases are colorless gases that are extremely un-reactive. One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. The thirty rare earth elements are composed of the lanthanide and actinide series. One element of the lanthan ...
... Noble Gases are colorless gases that are extremely un-reactive. One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. The thirty rare earth elements are composed of the lanthanide and actinide series. One element of the lanthan ...