Chapter 2. The Chemical Context of Life
... chemical behavior of an atom depends on number of electrons in its outermost shell ...
... chemical behavior of an atom depends on number of electrons in its outermost shell ...
Chapter 10: The Atom
... changed 1 kg of uranium into 1 kg of iron would release 130 trillion J of energy. ...
... changed 1 kg of uranium into 1 kg of iron would release 130 trillion J of energy. ...
chem_Atomic Structur..
... of the Electron: a) Cathode rays have identical properties regardless of the element used to produce them. All elements must contain identically charged electrons. b) Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons c) Electrons have ...
... of the Electron: a) Cathode rays have identical properties regardless of the element used to produce them. All elements must contain identically charged electrons. b) Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons c) Electrons have ...
The Fundamental Ideas in Chemistry
... Recall that chemical reactions can be represented by word equations or symbol equations. Describe how no atoms are lost or made during a chemical reaction so the mass of the products equals the mass of the reactants. Calculate the mass of a reactant or product from information about the masses of th ...
... Recall that chemical reactions can be represented by word equations or symbol equations. Describe how no atoms are lost or made during a chemical reaction so the mass of the products equals the mass of the reactants. Calculate the mass of a reactant or product from information about the masses of th ...
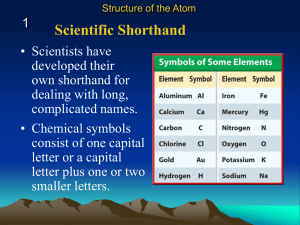
Bohr Diagrams, Metals, Non-Metals, and Metalloids
... to show the number and arrangement of electrons in each shell/energy level. ...
... to show the number and arrangement of electrons in each shell/energy level. ...
The Periodic Law
... What do you notice different about the first published periodic table and the now universally accepted periodic table? Find and write down the three main differences. ...
... What do you notice different about the first published periodic table and the now universally accepted periodic table? Find and write down the three main differences. ...
chem_Atomic Structure
... of the Electron: a) Cathode rays have identical properties regardless of the element used to produce them. All elements must contain identically charged electrons. b) Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons c) Electrons have ...
... of the Electron: a) Cathode rays have identical properties regardless of the element used to produce them. All elements must contain identically charged electrons. b) Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons c) Electrons have ...
Discovery of Atomic Structure
... Democritus’ ideas faded until the seventeenth century in Europe. Mullis ...
... Democritus’ ideas faded until the seventeenth century in Europe. Mullis ...
Periodic trends
... Group Trend • As you go down a group, you add energy levels • Outermost electrons not as attracted by the nucleus ...
... Group Trend • As you go down a group, you add energy levels • Outermost electrons not as attracted by the nucleus ...
Atoms and their structure
... – Earth - cool, heavy – Water - wet Blend these in different proportions to get all substances ...
... – Earth - cool, heavy – Water - wet Blend these in different proportions to get all substances ...
Atoms, Molecules and Ions
... · He discovered this by using alpha rays, which are charged, and therefore repelled by considerable electrical forces present in the nuclei of heavier atoms · Chadwick led the way to the starting of penetrating and splitting the nuclei of atoms. · Also led the way to the fission of uranium 235, whic ...
... · He discovered this by using alpha rays, which are charged, and therefore repelled by considerable electrical forces present in the nuclei of heavier atoms · Chadwick led the way to the starting of penetrating and splitting the nuclei of atoms. · Also led the way to the fission of uranium 235, whic ...
What is Chemistry?
... body. With such an enormous range of topics, it is essential to know about chemistry at some level in order to understand the world around us. ...
... body. With such an enormous range of topics, it is essential to know about chemistry at some level in order to understand the world around us. ...
Chapter 2 Powerpoint slides
... NUMBER IF NOT ON THE PERIODIC TABLE. • You will be given enough information to determine mass number or number of neutrons. ...
... NUMBER IF NOT ON THE PERIODIC TABLE. • You will be given enough information to determine mass number or number of neutrons. ...
Subatomic Particles
... Mass = 1.67 x 10-24g (1836x heavier than electron) • Charge = +1.76 x 108C (opposite electron) ...
... Mass = 1.67 x 10-24g (1836x heavier than electron) • Charge = +1.76 x 108C (opposite electron) ...
Identify the relationships among the components of the atom
... Note: It is incorrect to state that all atoms of a particular element are identical. While it is true that all atoms of a particular element have the same number of protons and electrons, the number of neutrons in their nuclei may differ. For example, there are three different types of potassium one ...
... Note: It is incorrect to state that all atoms of a particular element are identical. While it is true that all atoms of a particular element have the same number of protons and electrons, the number of neutrons in their nuclei may differ. For example, there are three different types of potassium one ...
Chapter6
... 1. Metals - most of the elements are metals (about 80%). Metals are good conductors of heat and electricity. They have luster or sheen due to their ability to reflect light. All metals are solid at room temperature except for mercury. Most metals are ductile (can be drawn into wire) and malleable (a ...
... 1. Metals - most of the elements are metals (about 80%). Metals are good conductors of heat and electricity. They have luster or sheen due to their ability to reflect light. All metals are solid at room temperature except for mercury. Most metals are ductile (can be drawn into wire) and malleable (a ...
Atomic Structure
... Bohr's model of the atom worked very well with hydrogen, which has only one electron per atom, however, problems arose when it was applied to atoms with more than one electron. ...
... Bohr's model of the atom worked very well with hydrogen, which has only one electron per atom, however, problems arose when it was applied to atoms with more than one electron. ...
Subatomic Particles
... › Positive Charge = loss of e-’s H becomes H+1 › Negative Charge = gaining of e-’s H becomes H-1 ...
... › Positive Charge = loss of e-’s H becomes H+1 › Negative Charge = gaining of e-’s H becomes H-1 ...
Chapter 4: Atomic Structure
... Bohr's model of the atom worked very well with hydrogen, which has only one electron per atom, however, problems arose when it was applied to atoms with more than one electron. ...
... Bohr's model of the atom worked very well with hydrogen, which has only one electron per atom, however, problems arose when it was applied to atoms with more than one electron. ...