Physical Science
... mixtures of isotopes. Isotopes are atoms of the same element that differ in the number of neutrons. ...
... mixtures of isotopes. Isotopes are atoms of the same element that differ in the number of neutrons. ...
UNIT VIII - St John Brebeuf
... 2. Elements become more metallic (or better metals) going down a family in the periodic table ...
... 2. Elements become more metallic (or better metals) going down a family in the periodic table ...
Early Atomic Theories
... • Dalton’s theory had to be revised as additional information was learned that could not be explained by his theory. ...
... • Dalton’s theory had to be revised as additional information was learned that could not be explained by his theory. ...
Station 1 - The Periodic Table, Molecules and Molecular
... 1. What is the distinction between atomic number and mass number? Between mass number and atomic mass? 2. Distinguish between the terms family and period in connection with the periodic table. For which of these is the term group also used. 3. When metals react with nonmetals, an ionic compound gene ...
... 1. What is the distinction between atomic number and mass number? Between mass number and atomic mass? 2. Distinguish between the terms family and period in connection with the periodic table. For which of these is the term group also used. 3. When metals react with nonmetals, an ionic compound gene ...
The chemical elements are fundamental building materials of matter
... • 1.A: All matter is made of atoms. There are a limited number of types of atoms: these are the elements. • 1.B: The atoms of each element have unique structures arising from interactions between electrons and nuclei. • 1.C: Elements display periodicity in their properties when the elements are orga ...
... • 1.A: All matter is made of atoms. There are a limited number of types of atoms: these are the elements. • 1.B: The atoms of each element have unique structures arising from interactions between electrons and nuclei. • 1.C: Elements display periodicity in their properties when the elements are orga ...
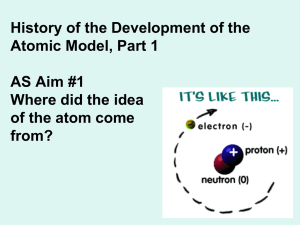
1.3 History of the Atom Notes
... by founding a law which we know today as Dalton's law of partial pressures. Dalton went further and asserted that all matter and not just gases must consist of small particles. Relating his atomic theory to Democritus, he used the name "atom" to name these tiny particles of matter. Dalton's atomic t ...
... by founding a law which we know today as Dalton's law of partial pressures. Dalton went further and asserted that all matter and not just gases must consist of small particles. Relating his atomic theory to Democritus, he used the name "atom" to name these tiny particles of matter. Dalton's atomic t ...
atom`s - Hauppauge School District
... Mechanical Model • Bohr’s shell model at the right is not quite right either! • Electrons actually exist in ________________________ around the nucleus, not in orbits like planets around the Sun • As per the Modern Atomic Model • Also known as the Wave Mechanical Model of the Atom ...
... Mechanical Model • Bohr’s shell model at the right is not quite right either! • Electrons actually exist in ________________________ around the nucleus, not in orbits like planets around the Sun • As per the Modern Atomic Model • Also known as the Wave Mechanical Model of the Atom ...
Atomic structure
... which was only a few atoms thick. they found that although most of them passed through. About 1 in 10,000 hit ...
... which was only a few atoms thick. they found that although most of them passed through. About 1 in 10,000 hit ...
Atoms of different elements are
... Use notation for writing isotope symbols: 235 or U-235 92 U Identify isotope using mass number and atomic number and relate to number of protons, neutrons and electrons Have a conceptual awareness of the nature of average atomic mass. (Relative abundance of each isotope determines the average- no ca ...
... Use notation for writing isotope symbols: 235 or U-235 92 U Identify isotope using mass number and atomic number and relate to number of protons, neutrons and electrons Have a conceptual awareness of the nature of average atomic mass. (Relative abundance of each isotope determines the average- no ca ...
Ch. 2: Biochemistry
... Element: substance that cannot be broken down to other substances by chemical reactions Compound: substance consisting of two or more elements in a fixed ratio Compound may not have same characteristics as elements it’s made of ...
... Element: substance that cannot be broken down to other substances by chemical reactions Compound: substance consisting of two or more elements in a fixed ratio Compound may not have same characteristics as elements it’s made of ...
History of atom
... The name atom comes from the Greek ´´átomos´´, which means uncuttable, or indivisible, something that cannot be divided further.The concept of an atom as an indivisible component of matter was first proposed by early Indian and Greek philosophers. In the 17th and 18th centuries, chemists provided a ...
... The name atom comes from the Greek ´´átomos´´, which means uncuttable, or indivisible, something that cannot be divided further.The concept of an atom as an indivisible component of matter was first proposed by early Indian and Greek philosophers. In the 17th and 18th centuries, chemists provided a ...
atom
... • Remember Rutherford’s planetary model? (a cloud of electrons, like planets, orbiting a small, compact nucleus of positive charge). Unfortunately, when electrical charge is involved, it gets more complicated. • First, planets do not have electric charge, and orbiting charged particles would lose en ...
... • Remember Rutherford’s planetary model? (a cloud of electrons, like planets, orbiting a small, compact nucleus of positive charge). Unfortunately, when electrical charge is involved, it gets more complicated. • First, planets do not have electric charge, and orbiting charged particles would lose en ...
atomic structure i
... People have difficulty with the size of an atom. It's so infinitesimally small that we neglect it. But if we could expand an atom's size to fit our visible world, we would find it impossible to ignore. If the nucleus of an atom were the size of a grape, the electrons would be about one mile away! Or ...
... People have difficulty with the size of an atom. It's so infinitesimally small that we neglect it. But if we could expand an atom's size to fit our visible world, we would find it impossible to ignore. If the nucleus of an atom were the size of a grape, the electrons would be about one mile away! Or ...
Review for Test
... 3. If Fred had two bar magnets, could he have placed them near the screen without causing any distortion in the image? Explain. __________________________________________________________________________________________________ _________________________________________________________________________ ...
... 3. If Fred had two bar magnets, could he have placed them near the screen without causing any distortion in the image? Explain. __________________________________________________________________________________________________ _________________________________________________________________________ ...
Atomic Theory
... radiation that knocked them free, thus measuring the mass of individual neutrons, and providing an explanation of why atoms were being ejected from a source of radiation previously undiscovered. IV. In 1932, he found definitive evidence for the existence of a neutral particle that had roughly the sa ...
... radiation that knocked them free, thus measuring the mass of individual neutrons, and providing an explanation of why atoms were being ejected from a source of radiation previously undiscovered. IV. In 1932, he found definitive evidence for the existence of a neutral particle that had roughly the sa ...
Chemistry 106: General Chemistry
... (32) If 4 mol of Neon gas and 6 mol of Krypton gas are contained in a flask whose total pressure is 1000 mmHg, the partial pressure of Neon gas is: (a) greater than the partial pressure of the Krypton gas (b) less than the partial pressure of the Krypton gas (c) the same as the partial pressure of t ...
... (32) If 4 mol of Neon gas and 6 mol of Krypton gas are contained in a flask whose total pressure is 1000 mmHg, the partial pressure of Neon gas is: (a) greater than the partial pressure of the Krypton gas (b) less than the partial pressure of the Krypton gas (c) the same as the partial pressure of t ...
atoms
... the US, each of its atoms would be only about 3 cm in diameter – about the size of a ping pong ball • A human hair is about 1 million carbon atoms wide • A typical human cell contains roughly 1 trillion atoms • A speck of dust might contain 3x1012 (3 trillion) atoms • It would take you around 500 ye ...
... the US, each of its atoms would be only about 3 cm in diameter – about the size of a ping pong ball • A human hair is about 1 million carbon atoms wide • A typical human cell contains roughly 1 trillion atoms • A speck of dust might contain 3x1012 (3 trillion) atoms • It would take you around 500 ye ...
Date: ______ Class: ______ Name
... Use the chart above to answer the following questions: 26. As the atomic mass increases the atomic number increases. 27. Does the atomic mass or atomic number increase at a higher rate? the atomic mass increases at a higher rate than the atomic number 28. As the protons increase the neutrons increas ...
... Use the chart above to answer the following questions: 26. As the atomic mass increases the atomic number increases. 27. Does the atomic mass or atomic number increase at a higher rate? the atomic mass increases at a higher rate than the atomic number 28. As the protons increase the neutrons increas ...
CH 5: The Atom
... • An orbital is the region of space where there is a high probability of finding an atom. • In the quantum mechanical atom, orbitals are arranged according to their size and shape. • The higher the energy of an orbital, the larger its ...
... • An orbital is the region of space where there is a high probability of finding an atom. • In the quantum mechanical atom, orbitals are arranged according to their size and shape. • The higher the energy of an orbital, the larger its ...
Electrons
... the elements in the second row (the second period) have two orbitals for their electrons. It goes down the periodic table like that. At this time, the maximum number of electron orbitals or electron shells for any element is seven. ...
... the elements in the second row (the second period) have two orbitals for their electrons. It goes down the periodic table like that. At this time, the maximum number of electron orbitals or electron shells for any element is seven. ...
The Atom - South Dade Senior High
... • JJ Thompson discovered one day that he two separate samples of neon gas, the same element, but they had different masses, how could this be? • The answer again is isotopes, if the number of neutrons is different the mass will be as well. ...
... • JJ Thompson discovered one day that he two separate samples of neon gas, the same element, but they had different masses, how could this be? • The answer again is isotopes, if the number of neutrons is different the mass will be as well. ...