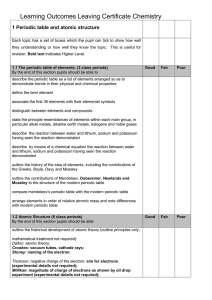
1 Periodic table and atomic structure
... Each topic has a set of boxes which the pupil can tick to show how well they understanding or how well they know the topic. This is useful for revision. Bold text indicates Higher Level. ...
... Each topic has a set of boxes which the pupil can tick to show how well they understanding or how well they know the topic. This is useful for revision. Bold text indicates Higher Level. ...
Atomic Masses
... Dalton’s atomic theory. Identify the parts of an atom, their location, charge, and relative mass. Determine the numbers of subatomic particles in an atom. ...
... Dalton’s atomic theory. Identify the parts of an atom, their location, charge, and relative mass. Determine the numbers of subatomic particles in an atom. ...
1 Periodic table and atomic structure
... Each topic has a set of boxes which the pupil can tick to show how well they understanding or how well they know the topic. This is useful for revision. Bold text indicates Higher Level. ...
... Each topic has a set of boxes which the pupil can tick to show how well they understanding or how well they know the topic. This is useful for revision. Bold text indicates Higher Level. ...
The Atom: From Philosophical Idea to Scientific Theory
... 6. An atom is electrically neutral because (a) neutrons balance the protons and electrons; (b) nuclear forces equalize the charges; (c) the number of protons and electrons is equal; (d) the number of protons and neutrons is equal. ...
... 6. An atom is electrically neutral because (a) neutrons balance the protons and electrons; (b) nuclear forces equalize the charges; (c) the number of protons and electrons is equal; (d) the number of protons and neutrons is equal. ...
BohrModels and Notation
... the middle of the field, the electron cloud is the rest of the field. ...
... the middle of the field, the electron cloud is the rest of the field. ...
Year 11 Chemistry: Chapter 3 ~ The Periodic Table
... Year 11 Chemistry: Chapter 3 ~ The Periodic Table 3.1 Why is that Periodic Table important? ________________________________________________________________ ________________________________________________________________ Mendeleev’s periodic table was an inspired development. Not only did it organi ...
... Year 11 Chemistry: Chapter 3 ~ The Periodic Table 3.1 Why is that Periodic Table important? ________________________________________________________________ ________________________________________________________________ Mendeleev’s periodic table was an inspired development. Not only did it organi ...
Ch 5 Notes
... columns, starting a new row each time the chemical properties repeated • Left blank spaces in his table, concluding that these spaces were elements that hadn’t been discovered yet. • Based on the patterns and the other elements around the blank space, he predicted the properties of those elements ...
... columns, starting a new row each time the chemical properties repeated • Left blank spaces in his table, concluding that these spaces were elements that hadn’t been discovered yet. • Based on the patterns and the other elements around the blank space, he predicted the properties of those elements ...
Chapter 4—Student Reading Parts of the atom http://www
... Another model of the hydrogen atom shows a cloudy-looking region in the space surrounding the nucleus. This model represents the electron as a cloud to show that it is not possible to know the exact location of an electron. The electron cloud shows the region surrounding the nucleus where the electr ...
... Another model of the hydrogen atom shows a cloudy-looking region in the space surrounding the nucleus. This model represents the electron as a cloud to show that it is not possible to know the exact location of an electron. The electron cloud shows the region surrounding the nucleus where the electr ...
Matter: The basics - Mrs. Mastin`s Website
... The electron cloud is where they are located and within that cloud are orbitals. Orbitals are categorized based on the amount of energy the electrons have. ...
... The electron cloud is where they are located and within that cloud are orbitals. Orbitals are categorized based on the amount of energy the electrons have. ...
Atoms Development of the Atomic Theory
... Beta radiation consists of fast-moving electrons. The symbol for a beta particle is B or 0B or 0e. They are small and fairly penetrating. The relative mass is 0 and the charge is -1. Gamma radiation (or gamma rays) is not particles but extremely high-energy electromagnetic radiation. They are very p ...
... Beta radiation consists of fast-moving electrons. The symbol for a beta particle is B or 0B or 0e. They are small and fairly penetrating. The relative mass is 0 and the charge is -1. Gamma radiation (or gamma rays) is not particles but extremely high-energy electromagnetic radiation. They are very p ...
Station 1: The Periodic Table 1a. Students know how to relate the
... Inonization energy (IE) is the energy required to remove an electron. o IE increases from left to right because the elements increase in nuclear charge in this direction. o IE decreases from top to bottom because as the atom’s shells get further away from the nucleus they are less attracted to it. ...
... Inonization energy (IE) is the energy required to remove an electron. o IE increases from left to right because the elements increase in nuclear charge in this direction. o IE decreases from top to bottom because as the atom’s shells get further away from the nucleus they are less attracted to it. ...
The Atom
... 3. A thin sheet of paper or clothing can stop alpha particles. It will not penetrate the skin on your body. 4. In alpha emission, the parent nuclide decays into a ...
... 3. A thin sheet of paper or clothing can stop alpha particles. It will not penetrate the skin on your body. 4. In alpha emission, the parent nuclide decays into a ...
AP Chemistry – Chapter 7 Reading Guide: Periodic Table of the
... 3. Define effective nuclear charge (Zeff). ...
... 3. Define effective nuclear charge (Zeff). ...
Atomic Structure
... The number of energy sublevels is the same as the number of the energy level (n) So, the 3rd energy level has 3 sublevels; the 5th energy level has 5 sublevels and so on. The sublevels are designated s, p, d, and f. ...
... The number of energy sublevels is the same as the number of the energy level (n) So, the 3rd energy level has 3 sublevels; the 5th energy level has 5 sublevels and so on. The sublevels are designated s, p, d, and f. ...
Periodic Table Study Guide
... Use a periodic table to answer the following questions: 17) Where are the metals located on the periodic table? 18) Where are the nonmetals located on the periodic table? 19) What classification of elements are between the metals and nonmetals on the periodic table? 20) What is the standard state f ...
... Use a periodic table to answer the following questions: 17) Where are the metals located on the periodic table? 18) Where are the nonmetals located on the periodic table? 19) What classification of elements are between the metals and nonmetals on the periodic table? 20) What is the standard state f ...
History of Atomic Theory Webquest
... Webquest: Atomic Theories and Models Answer these questions on your own, USING COMPLETE SENTENCES where appropriate (most of the questions, except tables and drawings). Atom Basics: Go to: http://www.chemtutor.com/struct.htm and read the “And you thought you were strange” section to answer the follo ...
... Webquest: Atomic Theories and Models Answer these questions on your own, USING COMPLETE SENTENCES where appropriate (most of the questions, except tables and drawings). Atom Basics: Go to: http://www.chemtutor.com/struct.htm and read the “And you thought you were strange” section to answer the follo ...
The Periodic Table and Periodic Law
... • Atomic radius is defined as half the distance between nuclei of identical atoms that are chemically bonded together. – Atomic Size/Radius decreases as you move left to right across the period… this is because there is an increased nuclear charge (more protons pulling the outer electrons closer to ...
... • Atomic radius is defined as half the distance between nuclei of identical atoms that are chemically bonded together. – Atomic Size/Radius decreases as you move left to right across the period… this is because there is an increased nuclear charge (more protons pulling the outer electrons closer to ...
intro to atoms
... electrons do not move about an atom in a definite path, like the planets around the sun. ...
... electrons do not move about an atom in a definite path, like the planets around the sun. ...
Atom
... •Atoms are mainly empty space •Nucleus of protons & neutrons •Electrons in empty space around nucleus ...
... •Atoms are mainly empty space •Nucleus of protons & neutrons •Electrons in empty space around nucleus ...
Structure of the Atom
... charge; therefore, protons must equal electrons. • For example: Sodium has 11 protons and 11 electrons. (The positives must equal the negatives. ) ...
... charge; therefore, protons must equal electrons. • For example: Sodium has 11 protons and 11 electrons. (The positives must equal the negatives. ) ...
Understanding the Atom
... English schoolteacher and scientist John Dalton did many experiments on gases that led to a new and more complete model of the atom. Dalton’s model had 5 major points. ...
... English schoolteacher and scientist John Dalton did many experiments on gases that led to a new and more complete model of the atom. Dalton’s model had 5 major points. ...























