Atoms, Molecules and Ions
... Atomic Mass • Naturally occurring chlorine is 75.78% 35Cl, which has an atomic mass of 34.969 amu, and 24.22% 37Cl, which has an atomic mass of 36.966 amu. Calculate the average atomic mass (that is, ...
... Atomic Mass • Naturally occurring chlorine is 75.78% 35Cl, which has an atomic mass of 34.969 amu, and 24.22% 37Cl, which has an atomic mass of 36.966 amu. Calculate the average atomic mass (that is, ...
Day 3
... periodic table. The guide will include a picture of an example block with information on each component (atomic number, atomic symbol, atomic mass). Explain -- Time Estimate: 20 minutes Each student will be assigned an element from the periodic table to make a poster about. The poster will include: ...
... periodic table. The guide will include a picture of an example block with information on each component (atomic number, atomic symbol, atomic mass). Explain -- Time Estimate: 20 minutes Each student will be assigned an element from the periodic table to make a poster about. The poster will include: ...
Chapter 2 Atoms and Elements
... Now using the mass of Hydrogen and Oxygen to show these results are consistent with the law of definite proportion ...
... Now using the mass of Hydrogen and Oxygen to show these results are consistent with the law of definite proportion ...
atomic mass
... Uses of Transition Elements • Most transition metals have higher melting points. • The filaments of light bulbs are made of tungsten, element 74. • Tungsten has the highest melting point of any metal (3,410°C) and will not melt when a current ...
... Uses of Transition Elements • Most transition metals have higher melting points. • The filaments of light bulbs are made of tungsten, element 74. • Tungsten has the highest melting point of any metal (3,410°C) and will not melt when a current ...
Chapter 2 Atoms and Ions
... radioactive methods, ought to begin a small research?" Now I had thought that, too, so I said, " Why not let him see if any alpha-particles can be scattered through a large angle?" I may tell you in confidence that I did not believe that they would be, since we knew the alpha-particle was a very fas ...
... radioactive methods, ought to begin a small research?" Now I had thought that, too, so I said, " Why not let him see if any alpha-particles can be scattered through a large angle?" I may tell you in confidence that I did not believe that they would be, since we knew the alpha-particle was a very fas ...
- Aboriginal Access to Engineering
... In the Periodic Table, 2 numbers appear beside each element. The whole number is the element’s atomic number and is equal to the number of protons it has. The other number is the element’s atomic mass. Neutrons and protons have almost exactly the same mass, so you can figure out the number of neutro ...
... In the Periodic Table, 2 numbers appear beside each element. The whole number is the element’s atomic number and is equal to the number of protons it has. The other number is the element’s atomic mass. Neutrons and protons have almost exactly the same mass, so you can figure out the number of neutro ...
Atomic mass - Cloudfront.net
... indivisible, spherical particles made up all of matter. He also argued that these particles were rearranged during a chemical reaction. ...
... indivisible, spherical particles made up all of matter. He also argued that these particles were rearranged during a chemical reaction. ...
Investigating Atoms and Atomic Theory
... electrons do not move about an atom in a definite path, like the planets around the sun. ...
... electrons do not move about an atom in a definite path, like the planets around the sun. ...
Lecture4
... unstable atomic nuclei release energetic subatomic particles. The word radioactivity is also used to refer to the subatomic particles themselves. This phenomenon is observed in the heavy elements, like uranium, and unstable isotopes, like carbon-14. For a better understanding of radioactivity, a rev ...
... unstable atomic nuclei release energetic subatomic particles. The word radioactivity is also used to refer to the subatomic particles themselves. This phenomenon is observed in the heavy elements, like uranium, and unstable isotopes, like carbon-14. For a better understanding of radioactivity, a rev ...
2 – Atomic Structure - Science at St. Dominics
... • Overall - Rutherford discovered that atoms had a nucleus, (a dense core of positive charge in the middle of the atom) using the alpha particle scattering experiment ...
... • Overall - Rutherford discovered that atoms had a nucleus, (a dense core of positive charge in the middle of the atom) using the alpha particle scattering experiment ...
Radioactivity
... unstable atomic nuclei release energetic subatomic particles. The word radioactivity is also used to refer to the subatomic particles themselves. This phenomenon is observed in the heavy elements, like uranium, and unstable isotopes, like carbon-14. For a better understanding of radioactivity, a rev ...
... unstable atomic nuclei release energetic subatomic particles. The word radioactivity is also used to refer to the subatomic particles themselves. This phenomenon is observed in the heavy elements, like uranium, and unstable isotopes, like carbon-14. For a better understanding of radioactivity, a rev ...
atomos
... electrons do not move about an atom in a definite path, like the planets around the sun. ...
... electrons do not move about an atom in a definite path, like the planets around the sun. ...
Chapter 05
... the symbol of the element, the mass number and the atomic number. Mass number Atomic number ...
... the symbol of the element, the mass number and the atomic number. Mass number Atomic number ...
Atom Notes - mcewenscience
... • Atoms of the same element (same number of protons) may have different numbers of neutrons. • These atoms are known as isotopes, and share similar chemical and physical properties • There are at least 2760 naturally occurring isotopes; tin has 38! ...
... • Atoms of the same element (same number of protons) may have different numbers of neutrons. • These atoms are known as isotopes, and share similar chemical and physical properties • There are at least 2760 naturally occurring isotopes; tin has 38! ...
Investigating Atoms and Atomic Theory
... electrons do not move about an atom in a definite path, like the planets around the sun. ...
... electrons do not move about an atom in a definite path, like the planets around the sun. ...
Investigating Atoms and Atomic Theory
... electrons do not move about an atom in a definite path, like the planets around the sun. ...
... electrons do not move about an atom in a definite path, like the planets around the sun. ...
atoms
... Bohr called the different orbits ‘shells’. The shells are called K,L,M,N etc. Electrons fill the lower shells and progress further from the nucleus. The number of electrons held by each shell is equal to 2n2 where n=number of shells. Under normal laboratory conditions, the electrons are in their gro ...
... Bohr called the different orbits ‘shells’. The shells are called K,L,M,N etc. Electrons fill the lower shells and progress further from the nucleus. The number of electrons held by each shell is equal to 2n2 where n=number of shells. Under normal laboratory conditions, the electrons are in their gro ...
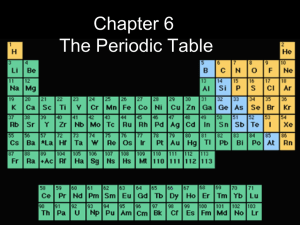
Chemistry 1 Chapter 4, The Periodic Table
... didn’t fit in the correct column by property when ordering them by atomic mass • he switched the order of these elements to group the elements in the column that matched their properties •at first he thought the atomic masses must have been incorrect, but they were correct, so Mendeleev couldn’t exp ...
... didn’t fit in the correct column by property when ordering them by atomic mass • he switched the order of these elements to group the elements in the column that matched their properties •at first he thought the atomic masses must have been incorrect, but they were correct, so Mendeleev couldn’t exp ...
Vocabulary List # 2 Covalent Bonding
... Attractive forces in which a hydrogen covalently bonded to a very electronegative atom is also weakly bonded to an unshared electron pair of another electronegative atom. ...
... Attractive forces in which a hydrogen covalently bonded to a very electronegative atom is also weakly bonded to an unshared electron pair of another electronegative atom. ...
Chemistry Midterm Review 2006
... These are topics from a traditional 1st quarter. If you used the thematic approach with me this year, you will notice that the topics are not in order of their presentation throughout the year. The final exam is cumulative so this review will help you with reviewing the concepts and calculations. No ...
... These are topics from a traditional 1st quarter. If you used the thematic approach with me this year, you will notice that the topics are not in order of their presentation throughout the year. The final exam is cumulative so this review will help you with reviewing the concepts and calculations. No ...
atomic mass - Bruder Chemistry
... How he explained it Atom is mostly empty Small dense, positive piece at center Alpha particles are deflected by it if they get close enough ...
... How he explained it Atom is mostly empty Small dense, positive piece at center Alpha particles are deflected by it if they get close enough ...
Atomic Theory Essay Research Paper In ancient
... Our first benefactor of atomic theory was John Dalton, a man later nick-named the “Father of atomic theory” for his contribution of many theories and laws to modern atomic theory. His theories answered many questions of skeptical scientists: elements combine with one another to form chemical compoun ...
... Our first benefactor of atomic theory was John Dalton, a man later nick-named the “Father of atomic theory” for his contribution of many theories and laws to modern atomic theory. His theories answered many questions of skeptical scientists: elements combine with one another to form chemical compoun ...
Basic Atomic Structure
... required to move electrons from one level to another. • unfortunately, this model only works for 1 atom—hydrogen. • Chadwick, James—“2 subatomic nucleus particles” model. • discovered the neutron in the nucleus • he used work previously done by Rutherford to establish the existence of the proton: • ...
... required to move electrons from one level to another. • unfortunately, this model only works for 1 atom—hydrogen. • Chadwick, James—“2 subatomic nucleus particles” model. • discovered the neutron in the nucleus • he used work previously done by Rutherford to establish the existence of the proton: • ...
06 Atomic Structure 2014
... detailed model with a central nucleus. He suggested that the positive charge was all in the central nucleus. This held the electrons in place by electrical attraction, so the electrons swarm around the nucleus. ...
... detailed model with a central nucleus. He suggested that the positive charge was all in the central nucleus. This held the electrons in place by electrical attraction, so the electrons swarm around the nucleus. ...