File - cpprashanths Chemistry
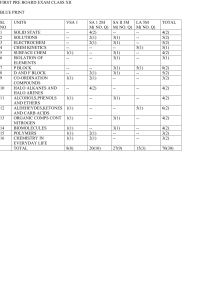
... 2. Question nos. 1 to 8 are very short answer questions and carry 1 mark each. 3. Question nos. 9 to 18 are short answer questions and carry 2 marks each. 4. Question nos. 19 to 27 are also short answer questions and carry 3 marks each 5. Question nos. 28 to 30 are long answer questions and carry 5 ...
... 2. Question nos. 1 to 8 are very short answer questions and carry 1 mark each. 3. Question nos. 9 to 18 are short answer questions and carry 2 marks each. 4. Question nos. 19 to 27 are also short answer questions and carry 3 marks each 5. Question nos. 28 to 30 are long answer questions and carry 5 ...
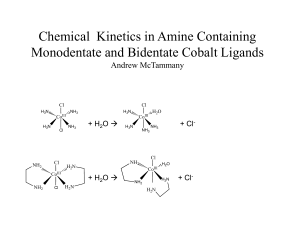
Chemical Kinetics in Monodentate and Bidentate Cobalt Compounds
... To elucidate the kinetic and thermodynamic quantities, the experiment needs to be repeated. The trans-Co(NH3)4Cl2 should be synthesized using a lower temperature and lower concentration of acid. A mixture of HCl and H2SO4 could be used instead. From there the effect of different ligands can be evalu ...
... To elucidate the kinetic and thermodynamic quantities, the experiment needs to be repeated. The trans-Co(NH3)4Cl2 should be synthesized using a lower temperature and lower concentration of acid. A mixture of HCl and H2SO4 could be used instead. From there the effect of different ligands can be evalu ...
Chapter+12
... What if we wanted 4 moles of water? What if we had 3 moles of oxygen, how much hydrogen would we need to react and how much water would we ...
... What if we wanted 4 moles of water? What if we had 3 moles of oxygen, how much hydrogen would we need to react and how much water would we ...
guess paper class xii
... Calculate the mass of a non-volatile solute (molecular mass 40) which should be dissolved in 114 gm octane to reduce its vapour pressure to 80%. 16 In a fuel cell (a device for producing electricity directly from chemical reaction) , methanol is used as fuel and oxygen gas is used as an oxidizer. Th ...
... Calculate the mass of a non-volatile solute (molecular mass 40) which should be dissolved in 114 gm octane to reduce its vapour pressure to 80%. 16 In a fuel cell (a device for producing electricity directly from chemical reaction) , methanol is used as fuel and oxygen gas is used as an oxidizer. Th ...
Cookies and Chemistry…Huh!?!?
... What if we wanted 4 moles of water? What if we had 3 moles of oxygen, how much hydrogen would we need to react and how much water would we ...
... What if we wanted 4 moles of water? What if we had 3 moles of oxygen, how much hydrogen would we need to react and how much water would we ...
2 - cloudfront.net
... How do you find out which is limited? 3. The chemical that makes the least amount of product is the “limiting reactant”. You can recognize limiting reactant problems because they will give you 2 amounts of chemical 4. Do two stoichiometry problems, one for each reactant given ...
... How do you find out which is limited? 3. The chemical that makes the least amount of product is the “limiting reactant”. You can recognize limiting reactant problems because they will give you 2 amounts of chemical 4. Do two stoichiometry problems, one for each reactant given ...
LaBrake, Fundamentals Diagnostic Questions
... c) iodine, I d) oxygen, O e) potassium, K 40. Which of the following common names is not correctly matched with the formula? a) NH3, ammonia b) H2O, water c) C2H2, dicarbon dihyrdide (correct) d) C2H4, ethylene e) N2O, nitrous oxide 41. Which has more molecules: a mole of H2O or a mole of O2? a) a m ...
... c) iodine, I d) oxygen, O e) potassium, K 40. Which of the following common names is not correctly matched with the formula? a) NH3, ammonia b) H2O, water c) C2H2, dicarbon dihyrdide (correct) d) C2H4, ethylene e) N2O, nitrous oxide 41. Which has more molecules: a mole of H2O or a mole of O2? a) a m ...
Adsorption and desorption
... But: Magnitude of ΔHad alone is not a sufficient criterion for the distinction: Change of geom. and electron. structure possible. Potential curve for the dissociative adsorption of a B2 molecule on a surface (Masel fig. 3.8, p.119.) Potential curves for (a) pure molecular adsorption, (b) activated d ...
... But: Magnitude of ΔHad alone is not a sufficient criterion for the distinction: Change of geom. and electron. structure possible. Potential curve for the dissociative adsorption of a B2 molecule on a surface (Masel fig. 3.8, p.119.) Potential curves for (a) pure molecular adsorption, (b) activated d ...
4 Types of Chemical Reactions and Solution Stoichiometry
... To understand the chemistry that occurs in such diverse places as the human body, the groundwater, the oceans, the local water treatment plant, your hair as you shampoo it, and so on, we must understand how substances dissolved in water react with each other. However, before we can understand soluti ...
... To understand the chemistry that occurs in such diverse places as the human body, the groundwater, the oceans, the local water treatment plant, your hair as you shampoo it, and so on, we must understand how substances dissolved in water react with each other. However, before we can understand soluti ...
Transition Metal-Modified Zirconium Phosphate Electrocatalysts for
... intercalated systems and that the metal‐adsorbed catalyst had retained α‐ZrP characteristics. In the metal-intercalated systems and that the metal-adsorbed catalyst had retained α-ZrP characteristics. case of pure α‐ZrP (Figure 3) two weight losses are observed, dehydration of water w ...
... intercalated systems and that the metal‐adsorbed catalyst had retained α‐ZrP characteristics. In the metal-intercalated systems and that the metal-adsorbed catalyst had retained α-ZrP characteristics. case of pure α‐ZrP (Figure 3) two weight losses are observed, dehydration of water w ...
Fall 2006
... (2 pts) Molecules of a liquid can pass into the vapor phase only if the a) liquid has little surface tension. b) vapor pressure of the liquid is high. c) temperature of the liquid is near its boiling point. d) molecules have sufficient kinetic energy to overcome the intermolecular forces in the liqu ...
... (2 pts) Molecules of a liquid can pass into the vapor phase only if the a) liquid has little surface tension. b) vapor pressure of the liquid is high. c) temperature of the liquid is near its boiling point. d) molecules have sufficient kinetic energy to overcome the intermolecular forces in the liqu ...
IIT-JEE (Advanced) - Brilliant Public School Sitamarhi
... Q.15 A sample containing only CaCO3 and MgCO3 is ignited to CaO and MgO. The mixture of oxides produced weight exactly half as much as the original sample. Calculate the percentages of CaCO3 and MgCO3 in the sample. Q.16 Determine the percentage composition of a mixture of anhydrous sodium carbonate ...
... Q.15 A sample containing only CaCO3 and MgCO3 is ignited to CaO and MgO. The mixture of oxides produced weight exactly half as much as the original sample. Calculate the percentages of CaCO3 and MgCO3 in the sample. Q.16 Determine the percentage composition of a mixture of anhydrous sodium carbonate ...
Unit 8 Chemical Equilibrium Focusing on Acid
... When small pieces of zinc are added to a dilute solution of excess hydrochloric acid in an open beaker (Figure 2), a vigorous reaction occurs with lots of gas and heat given off. The zinc continues to react and, when it is completely consumed, the visible signs of reaction come to an end. After seei ...
... When small pieces of zinc are added to a dilute solution of excess hydrochloric acid in an open beaker (Figure 2), a vigorous reaction occurs with lots of gas and heat given off. The zinc continues to react and, when it is completely consumed, the visible signs of reaction come to an end. After seei ...
Complete Solution Manual
... Utilizing the standard potentials in Table 17.1, E ocell = 0.40 V + 0.83 V = 1.23 V for the hydrogen-oxygen fuel cell. As with all fuel cells, the H2(g) and O2(g) reactants are continuously supplied. See Figure 17.16 for a schematic of this fuel cell. ...
... Utilizing the standard potentials in Table 17.1, E ocell = 0.40 V + 0.83 V = 1.23 V for the hydrogen-oxygen fuel cell. As with all fuel cells, the H2(g) and O2(g) reactants are continuously supplied. See Figure 17.16 for a schematic of this fuel cell. ...
chemistry - Brilliant Public School Sitamarhi
... in one unit cell of this mineral there are 4 Ca2+ ions and 8F– ions and that Ca2+ ions are arranged in a fcc lattice. The F– ions fill all the tetrahedral holes in the fcc lattice of Ca2+ ions. The edge of the unit cell is 5.46 × 10–8 cm in length. The density of the solid is 3.18 g cm–3. Use this i ...
... in one unit cell of this mineral there are 4 Ca2+ ions and 8F– ions and that Ca2+ ions are arranged in a fcc lattice. The F– ions fill all the tetrahedral holes in the fcc lattice of Ca2+ ions. The edge of the unit cell is 5.46 × 10–8 cm in length. The density of the solid is 3.18 g cm–3. Use this i ...
Polyhedral Oligomeric Silsesquioxane
... onto mesoporous silica particles because of the strong affinity of thiol to Pd2þ ions and Pd(0) NPs.39,40 Typically, there are four raw materials—metal salts, reductants, stabilizer, and phase-transfer agents—that are required for the chemical synthesis of metal NPs. Using a hydrophobic stabilizer, a ...
... onto mesoporous silica particles because of the strong affinity of thiol to Pd2þ ions and Pd(0) NPs.39,40 Typically, there are four raw materials—metal salts, reductants, stabilizer, and phase-transfer agents—that are required for the chemical synthesis of metal NPs. Using a hydrophobic stabilizer, a ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.