File
... production; HNO3: important industrial chemical, used to form nitrogen-based explosives, strong acid and a very strong oxidizing agent. ...
... production; HNO3: important industrial chemical, used to form nitrogen-based explosives, strong acid and a very strong oxidizing agent. ...
CHAPTER VI Fischer-Tropsch Refineries
... Initially, in 1938, the octane value was improved by mixing it with aromatics and alcohol, but later Ruhrchemie added two True-Vapour-Phase processes h with gas recycle to convert the higher boiling fractions to gasoline with a much higher octane number, for example the 30165°C fraction had a MON of ...
... Initially, in 1938, the octane value was improved by mixing it with aromatics and alcohol, but later Ruhrchemie added two True-Vapour-Phase processes h with gas recycle to convert the higher boiling fractions to gasoline with a much higher octane number, for example the 30165°C fraction had a MON of ...
Chapter 3
... in the Manufacture of Drugs Stoichiometry refers to the quantitative relationships between the substances that are consumed and produced by chemical reactions. These quantitative relationships are important in the development of large-scale production of such things as chemotherapeutic drugs for the ...
... in the Manufacture of Drugs Stoichiometry refers to the quantitative relationships between the substances that are consumed and produced by chemical reactions. These quantitative relationships are important in the development of large-scale production of such things as chemotherapeutic drugs for the ...
Chapter 4
... chapter, we will discuss three major categories of reactions that occur in aqueous solutions: precipitation reactions, acid-base reactions, and redox reactions. In later chapters, we will study the structural characteristics and properties of water—the so-called universal solvent— and its solutions. ...
... chapter, we will discuss three major categories of reactions that occur in aqueous solutions: precipitation reactions, acid-base reactions, and redox reactions. In later chapters, we will study the structural characteristics and properties of water—the so-called universal solvent— and its solutions. ...
Mole Concept
... In some problems, amounts of more than one species are given. In that case your first task is to determine which species is the limiting reagent. Just as you can make only 1 bicycle from 2 wheels and 4 handlebars (with 3 handlebars left over), and only 2 bicycles from 8 wheels and 2 handlebars (with ...
... In some problems, amounts of more than one species are given. In that case your first task is to determine which species is the limiting reagent. Just as you can make only 1 bicycle from 2 wheels and 4 handlebars (with 3 handlebars left over), and only 2 bicycles from 8 wheels and 2 handlebars (with ...
Chapter 9 Stoichiometry
... How many grams of CaF2 is formed when 35.8mL of 0.678 M NaF is treated with excess of Ca(NO3)2(aq)? NaF + Ca(NO3)2 CaF2 + Na2(NO3)2 ...
... How many grams of CaF2 is formed when 35.8mL of 0.678 M NaF is treated with excess of Ca(NO3)2(aq)? NaF + Ca(NO3)2 CaF2 + Na2(NO3)2 ...
Unit 6 Review Answers
... considered a modernized Arrhenius acid, however.) Ammonia can’t be an Arrhenius base, because it does not contain hydroxide ions. (Ammonia could be considered a modernized Arrhenius base, however.) Students should disagree. A solution with a pH of 4 has [H3O+] = 0.0001 mol/L, whereas a solution with ...
... considered a modernized Arrhenius acid, however.) Ammonia can’t be an Arrhenius base, because it does not contain hydroxide ions. (Ammonia could be considered a modernized Arrhenius base, however.) Students should disagree. A solution with a pH of 4 has [H3O+] = 0.0001 mol/L, whereas a solution with ...
Atmospheric Formation_TELTEK
... Scheme 2.5. Possible trimethylamine gas phase chemistry, leading to N2O or HCN. (From Schade and Crutzen, Ref. 17). In the exploratory (CH3)3N photo-oxidation study by Pitts et al.20 the aerosol formed contained ca. 3 µg m-3 (1.6 ppb) CHONH2 (formamide) and another amide-like compound with M=87 was ...
... Scheme 2.5. Possible trimethylamine gas phase chemistry, leading to N2O or HCN. (From Schade and Crutzen, Ref. 17). In the exploratory (CH3)3N photo-oxidation study by Pitts et al.20 the aerosol formed contained ca. 3 µg m-3 (1.6 ppb) CHONH2 (formamide) and another amide-like compound with M=87 was ...
Chemistry II - Mr. Dougan`s Wonderful World of Chemistry
... 3. Record your results in the spaces provided on your data table. If you do not observe a change, record NR or No Reaction. But if a change is observed, report both the color and the results of each of the reactions. At this point there are two choices: the mixture is either clear or opaque. An opaq ...
... 3. Record your results in the spaces provided on your data table. If you do not observe a change, record NR or No Reaction. But if a change is observed, report both the color and the results of each of the reactions. At this point there are two choices: the mixture is either clear or opaque. An opaq ...
The shock tube as wave reactor for kinetic studies and material
... providing nearly instantaneous and uniform heating of reactants, it allows rapid quenching of products leading to particle condensation and growth. The effect of varying initial temperature, pressure, and mixture composition on the size and yield of the particles produced, can be conveniently studie ...
... providing nearly instantaneous and uniform heating of reactants, it allows rapid quenching of products leading to particle condensation and growth. The effect of varying initial temperature, pressure, and mixture composition on the size and yield of the particles produced, can be conveniently studie ...
Tro Chemistry a Molecular Approach, 3E
... (P, V, and T) defines the state (or macrostate) of the system. As long as these conditions remain constant, the energy of the system also remains constant. However, exactly where that energy is at any given instant is anything but constant. At any one instant, a particular gas particle may have lots ...
... (P, V, and T) defines the state (or macrostate) of the system. As long as these conditions remain constant, the energy of the system also remains constant. However, exactly where that energy is at any given instant is anything but constant. At any one instant, a particular gas particle may have lots ...
ExamView - 1984 AP Chemistry Exam.tst
... (1) Test Questions are Copyright © 1984-2002 by College Entrance Examination Board, Princeton, NJ. All rights reserved. For face-to-face teaching purposes, classroom teachers are permitted to reproduce the questions. Web or Mass distribution prohibited. (2) AP® is a registered trademark of the Colle ...
... (1) Test Questions are Copyright © 1984-2002 by College Entrance Examination Board, Princeton, NJ. All rights reserved. For face-to-face teaching purposes, classroom teachers are permitted to reproduce the questions. Web or Mass distribution prohibited. (2) AP® is a registered trademark of the Colle ...
sample chapter
... Many chemical reactions and virtually all biological processes take place in an aqueous environment. Therefore, it is important to understand the properties of different substances in solution with water. To start with, what exactly is a solution? A solution is a homogeneous mixture of two or more s ...
... Many chemical reactions and virtually all biological processes take place in an aqueous environment. Therefore, it is important to understand the properties of different substances in solution with water. To start with, what exactly is a solution? A solution is a homogeneous mixture of two or more s ...
Chapter 3 Stoichiometry: Calculations with Chemical
... – %C is determined from the mass of CO2 produced – %H is determined from the mass of H2O produced – %O is determined by difference after the C and H have Stoichiometry ...
... – %C is determined from the mass of CO2 produced – %H is determined from the mass of H2O produced – %O is determined by difference after the C and H have Stoichiometry ...
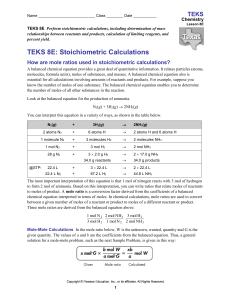
TEKS 5 - Online Learning Exchange
... Before the reaction takes place, nitrogen and hydrogen are present in a 2:3 molecule (mole) ratio. The reaction takes place according to the balanced equation. One molecule (mole) of N2 reacts with three molecules (moles) of H2 to produce two molecules (moles) of NH3. At this point, all the hydrogen ...
... Before the reaction takes place, nitrogen and hydrogen are present in a 2:3 molecule (mole) ratio. The reaction takes place according to the balanced equation. One molecule (mole) of N2 reacts with three molecules (moles) of H2 to produce two molecules (moles) of NH3. At this point, all the hydrogen ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.