Instructor`s Resource Manual
... context in which the word occurred. All of these important terms are included in the glossary at the end of the book. Problem solving has received special attention in the text. Students should be aware that key statements or equations used for problem solving are highlighted. Also, page numbers of ...
... context in which the word occurred. All of these important terms are included in the glossary at the end of the book. Problem solving has received special attention in the text. Students should be aware that key statements or equations used for problem solving are highlighted. Also, page numbers of ...
Thermochemistry - hrsbstaff.ednet.ns.ca
... The law of conservation of energy states that the total energy of the universe is constant. In other words, energy can be neither destroyed nor created. This idea can be expressed by the following equation: ∆Euniverse = 0 Energy can, however, be transferred from one substance to another. It can also ...
... The law of conservation of energy states that the total energy of the universe is constant. In other words, energy can be neither destroyed nor created. This idea can be expressed by the following equation: ∆Euniverse = 0 Energy can, however, be transferred from one substance to another. It can also ...
Objectives - hartman
... Conversions of Quantities in Moles, continued Sample Problem A In a spacecraft, the carbon dioxide exhaled by astronauts can be removed by its reaction with lithium hydroxide, LiOH, according to the following chemical equation. CO2(g) + 2LiOH(s) → Li2CO3(s) + H2O(l) How many moles of lithium hydroxi ...
... Conversions of Quantities in Moles, continued Sample Problem A In a spacecraft, the carbon dioxide exhaled by astronauts can be removed by its reaction with lithium hydroxide, LiOH, according to the following chemical equation. CO2(g) + 2LiOH(s) → Li2CO3(s) + H2O(l) How many moles of lithium hydroxi ...
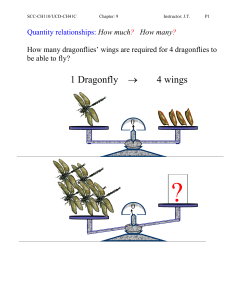
Quantity relationships: How much
... ignited. Water forms according to the following combination reaction: 2H2(g) +O2(g) → 2H2O(g) Which reactant is limiting? How much water will the reaction produce? ...
... ignited. Water forms according to the following combination reaction: 2H2(g) +O2(g) → 2H2O(g) Which reactant is limiting? How much water will the reaction produce? ...
MULTIPLY CHOICE QUESTIONS ON MEDICAL CHEMISTRY
... reaction NH3 + HCl = NH4Cl is 0.25 mol/L, in 0.2s it changes into 0.25 mol/L. What is the average rate of this reaction? А. 0,1 mol/ Lּs B. 0,2 mol/ Lּs C. 2 mol/ Lּs D. 0,02 mol/ Lּs E. 0,04 mol/ Lּs 2.30. How will change the rate of reaction 3H2 + N2 ⇆ 2NH3, if the volume of systhem decrease in tw ...
... reaction NH3 + HCl = NH4Cl is 0.25 mol/L, in 0.2s it changes into 0.25 mol/L. What is the average rate of this reaction? А. 0,1 mol/ Lּs B. 0,2 mol/ Lּs C. 2 mol/ Lּs D. 0,02 mol/ Lּs E. 0,04 mol/ Lּs 2.30. How will change the rate of reaction 3H2 + N2 ⇆ 2NH3, if the volume of systhem decrease in tw ...
Stoichiometry and the Mole
... mole is a lot of things—but atoms and molecules are very tiny. One mole of carbon atoms would make a cube that is 1.74 cm on a side, small enough to carry in your pocket. Why is the mole unit so important? It represents the link between the microscopic and the macroscopic, especially in terms of mas ...
... mole is a lot of things—but atoms and molecules are very tiny. One mole of carbon atoms would make a cube that is 1.74 cm on a side, small enough to carry in your pocket. Why is the mole unit so important? It represents the link between the microscopic and the macroscopic, especially in terms of mas ...
Stoichiometery
... Real Chemistry is all about doing chemical reactions. Chemistry is about making or breaking bonds in order to rearrange atoms and make new compounds. ...
... Real Chemistry is all about doing chemical reactions. Chemistry is about making or breaking bonds in order to rearrange atoms and make new compounds. ...
Calculations with Chemical Formulas and Equations
... – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been Stoichiometry determined ...
... – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been Stoichiometry determined ...
National German competition
... To become a member of the German IChO-team you have to be successful in four rounds. The problems to be solved in the 1st round are sent to all highschools. To solve the problems the students may use all resources available, e.g. textbooks etc. All the students who solve about 70% will receive the p ...
... To become a member of the German IChO-team you have to be successful in four rounds. The problems to be solved in the 1st round are sent to all highschools. To solve the problems the students may use all resources available, e.g. textbooks etc. All the students who solve about 70% will receive the p ...
RUMPLE-DISSERTATION-2014 - SMARTech Home
... Eckert. The opportunity to work with such skilled scientists and kind mentors is a rare one, and I am extremely glad I had the opportunity to learn from them. I have always been in awe of their brilliance (I’m pretty sure they have each forgotten more than I’ll ever learn, and they still know so muc ...
... Eckert. The opportunity to work with such skilled scientists and kind mentors is a rare one, and I am extremely glad I had the opportunity to learn from them. I have always been in awe of their brilliance (I’m pretty sure they have each forgotten more than I’ll ever learn, and they still know so muc ...
Stoichiometry
... • When balancing an equation, ONLY the coefficients can be changed. • NEVER change the subscripts. • For example: 3H2O 3 is the coefficient. 2 and 1 are the subscripts. • Changing the subscripts changes the compound. H2O2 is not water but hydrogen peroxide. ...
... • When balancing an equation, ONLY the coefficients can be changed. • NEVER change the subscripts. • For example: 3H2O 3 is the coefficient. 2 and 1 are the subscripts. • Changing the subscripts changes the compound. H2O2 is not water but hydrogen peroxide. ...
Specification – AS/A Level Chemistry A
... (ii) molecular formula as the actual number of atoms of each element in a molecule; (d) calculate empirical and molecular formulae, ...
... (ii) molecular formula as the actual number of atoms of each element in a molecule; (d) calculate empirical and molecular formulae, ...
Stoichiometry
... Mole Ratio – the ratio of moles of one substance to moles of another substance in a balanced chemical equation The coefficients in a balanced equation give the relative numbers of molecules, as well as, the relative number of moles. ...
... Mole Ratio – the ratio of moles of one substance to moles of another substance in a balanced chemical equation The coefficients in a balanced equation give the relative numbers of molecules, as well as, the relative number of moles. ...
National German Competition and Problems of the IChO
... d) Calculate the mass concentration of calcium in mg/L! e) Which water supply station delivered the water? From hearsay Eileen knows that salty water is particularly healthy. She wants to raise the mass content of chloride in the pool water to 1%. 1 kg of pure salt costs €1.24. The pool has a base a ...
... d) Calculate the mass concentration of calcium in mg/L! e) Which water supply station delivered the water? From hearsay Eileen knows that salty water is particularly healthy. She wants to raise the mass content of chloride in the pool water to 1%. 1 kg of pure salt costs €1.24. The pool has a base a ...
Stoichiometry and the Mole - 2012 Book Archive
... pound of flour, and 1 pound of sugar. (That’s why it’s called “pound cake.”) If you have 4 pounds of butter, how many pounds of sugar, flour, and eggs do you need? You would need 4 pounds each of sugar, flour, and eggs. Now suppose you have 1.00 g H2. If the chemical reaction follows the balanced ch ...
... pound of flour, and 1 pound of sugar. (That’s why it’s called “pound cake.”) If you have 4 pounds of butter, how many pounds of sugar, flour, and eggs do you need? You would need 4 pounds each of sugar, flour, and eggs. Now suppose you have 1.00 g H2. If the chemical reaction follows the balanced ch ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.








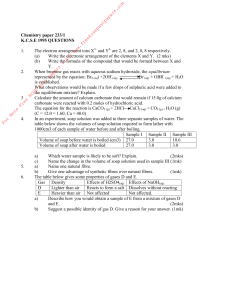



![1 Solutions 4a (Chapter 4 problems) Chem151 [Kua]](http://s1.studyres.com/store/data/002731518_1-574ec10e88e667508364281b6325aeef-300x300.png)