![Preparation and Inner-sphere Oxidation of Ternary Iminodiacetato Chromium [III]](http://s1.studyres.com/store/data/008844767_1-9b02a033035d53dea970333df8a85c48-300x300.png)
Preparation and Inner-sphere Oxidation of Ternary Iminodiacetato Chromium [III]
... Ternary metal complexes might appear in biological fluids creating specific structure[1-3], most frequently manifesting themselves as enzyme-metal ionsubstrate complexes[3-6]. This explains why ternary system has recently received increasing attention. Chromium (III) complexes with nucleotides are u ...
... Ternary metal complexes might appear in biological fluids creating specific structure[1-3], most frequently manifesting themselves as enzyme-metal ionsubstrate complexes[3-6]. This explains why ternary system has recently received increasing attention. Chromium (III) complexes with nucleotides are u ...
View/Open
... As a consequence of these reactivity differences, it’s usually possible to convert a more reactive acid derivative into a less reactive one. Acid chlorides, for instance, can be directly converted into anhydrides, thioesters, esters, and amides, but amides can’t be directly converted into esters, th ...
... As a consequence of these reactivity differences, it’s usually possible to convert a more reactive acid derivative into a less reactive one. Acid chlorides, for instance, can be directly converted into anhydrides, thioesters, esters, and amides, but amides can’t be directly converted into esters, th ...
mole ratio
... moles of aluminum oxide. • In other words, we produce half as much aluminum oxide as aluminum with which we start. • We produced 0.1205 mol Al2O3 from 0.241 mol Al, so it works! ...
... moles of aluminum oxide. • In other words, we produce half as much aluminum oxide as aluminum with which we start. • We produced 0.1205 mol Al2O3 from 0.241 mol Al, so it works! ...
Cubic SiC Nanowires: Growth, Characterization and
... function of the etching time (b).The silicon dioxide and the silicon carbide related emissions show different decays. the etching time, while the SiC related emissions showed a linear decrease (Fig. 8b). The SiO2 related emissions decrease in intensity due to material removal. On the contrary, the c ...
... function of the etching time (b).The silicon dioxide and the silicon carbide related emissions show different decays. the etching time, while the SiC related emissions showed a linear decrease (Fig. 8b). The SiO2 related emissions decrease in intensity due to material removal. On the contrary, the c ...
Chemistry - A Quantitative Science
... Chemistry - A Molecular Science (CAMS), the first half of this two-volume sequence, stressed bonding, structure, and reactivity. The material was qualitative and stressed several types of reactions and the factors that affected their relative extents of reaction. However, as the title of this text s ...
... Chemistry - A Molecular Science (CAMS), the first half of this two-volume sequence, stressed bonding, structure, and reactivity. The material was qualitative and stressed several types of reactions and the factors that affected their relative extents of reaction. However, as the title of this text s ...
Stoichiometry
... Chemistry - A Molecular Science (CAMS), the first half of this two-volume sequence, stressed bonding, structure, and reactivity. The material was qualitative and stressed several types of reactions and the factors that affected their relative extents of reaction. However, as the title of this text s ...
... Chemistry - A Molecular Science (CAMS), the first half of this two-volume sequence, stressed bonding, structure, and reactivity. The material was qualitative and stressed several types of reactions and the factors that affected their relative extents of reaction. However, as the title of this text s ...
Chemical Vapor Deposition (CVD)
... • This technique is often used in many thin film applications. • By varying the experimental conditions—substrate material, substrate temperature, composition of the reaction gas mixture, total pressure gas flows, etc.— materials with different properties can be grown. ...
... • This technique is often used in many thin film applications. • By varying the experimental conditions—substrate material, substrate temperature, composition of the reaction gas mixture, total pressure gas flows, etc.— materials with different properties can be grown. ...
LABORATORY MANUAL FOR CHEMISTRY 102
... In part D of this experiment how did the presence of the copper(II) nitrate affect the rate of the reaction? ...
... In part D of this experiment how did the presence of the copper(II) nitrate affect the rate of the reaction? ...
Application of Novel Phosphine Ligands in Palladium
... The complex reacts with substrate B to give the product D while releasing the catalyst C. This sequence consisting of the generation of the substrate-catalyst-complex, the product formation and the release of the catalyst is called a catalytic cycle. After the catalyst is released, it can reenter th ...
... The complex reacts with substrate B to give the product D while releasing the catalyst C. This sequence consisting of the generation of the substrate-catalyst-complex, the product formation and the release of the catalyst is called a catalytic cycle. After the catalyst is released, it can reenter th ...
PX312-1718
... 17. Which of Figures I–IV represent(s) the result of mixing aqueous solutions of Na2S and NiCl2 in which the ion product Qc > Ksp for the insoluble product? (C = cation, A = anion) ...
... 17. Which of Figures I–IV represent(s) the result of mixing aqueous solutions of Na2S and NiCl2 in which the ion product Qc > Ksp for the insoluble product? (C = cation, A = anion) ...
PHYSICAL CHEMISTRY IN BRIEF
... The Physical Chemistry In Brief offers a digest of all major formulas, terms and definitions needed for an understanding of the subject. They are illustrated by schematic figures, simple worked-out examples, and a short accompanying text. The concept of the book makes it different from common univer ...
... The Physical Chemistry In Brief offers a digest of all major formulas, terms and definitions needed for an understanding of the subject. They are illustrated by schematic figures, simple worked-out examples, and a short accompanying text. The concept of the book makes it different from common univer ...
2008 Equilibrium -- without math (PowerPoint 13 MB)
... • The value of k also depends on how the equilibrium equation is balanced. (If you double the chemical equation throughout, the corresponding equilibrium constant will be the square of the original value.) Equilibrium 2007-2008 ...
... • The value of k also depends on how the equilibrium equation is balanced. (If you double the chemical equation throughout, the corresponding equilibrium constant will be the square of the original value.) Equilibrium 2007-2008 ...
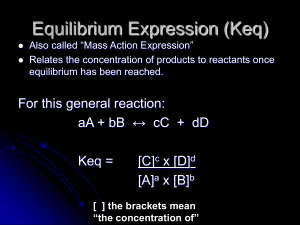
Equilibrium Expression (Keq)
... (The is a unitless number and is unique to that reaction.) The only thing that can change the value of Keq is a change in temperature. ...
... (The is a unitless number and is unique to that reaction.) The only thing that can change the value of Keq is a change in temperature. ...
To do List
... exchange of anions and cations between two compounds; for example, AgNO3(aq) + KI(aq) -----> KNO(aq) + AgI(s) ...
... exchange of anions and cations between two compounds; for example, AgNO3(aq) + KI(aq) -----> KNO(aq) + AgI(s) ...
Elementary Steps, the Role of Chemisorbed Oxygen, and the Effects
... but rate constants are significantly larger than those on other metals and cause C!H bond dissociation to become reversible. Fast C!H bond activation on Pd also leads to kinetically detectable coverages by C* and H* intermediates, which inhibit reforming reactions.27 In CH4!O2 mixtures, O*!* and O*!O ...
... but rate constants are significantly larger than those on other metals and cause C!H bond dissociation to become reversible. Fast C!H bond activation on Pd also leads to kinetically detectable coverages by C* and H* intermediates, which inhibit reforming reactions.27 In CH4!O2 mixtures, O*!* and O*!O ...
Preparatory Problems of the 40th IChO - IChO-2016
... alone, however, half asleep, with his long, thin form curled up in the recesses of his arm-chair. A formidable array of bottles and test-tubes, with the pungent smell of hydrochloric acid, told me that he had spent his day in the chemical work which was so dear to him. It was obvious to me that my c ...
... alone, however, half asleep, with his long, thin form curled up in the recesses of his arm-chair. A formidable array of bottles and test-tubes, with the pungent smell of hydrochloric acid, told me that he had spent his day in the chemical work which was so dear to him. It was obvious to me that my c ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.