Chemistry: Percent Yield
... 10: 3.3a In all chemical reactions there is a conservation of mass, energy, and charge. 17: 3.1cc A compound is a substance composed of two or more different elements that are chemically combined in a fixed proportion. A chemical compound can be broken down by chemical means. A chemical compound can ...
... 10: 3.3a In all chemical reactions there is a conservation of mass, energy, and charge. 17: 3.1cc A compound is a substance composed of two or more different elements that are chemically combined in a fixed proportion. A chemical compound can be broken down by chemical means. A chemical compound can ...
chemistry - Textbooks Online
... form a molecule" is required to gain knowledge of the followingi) to know about how atoms of same element form different compounds combining with different elements. ii) to know why particular shapes are adopted by molecules. iii) to understand the specific properties of molecules or ions and the re ...
... form a molecule" is required to gain knowledge of the followingi) to know about how atoms of same element form different compounds combining with different elements. ii) to know why particular shapes are adopted by molecules. iii) to understand the specific properties of molecules or ions and the re ...
Stoichiometry - coercingmolecules
... b. Consider a 500.-mg tablet. How many moles of sodium ascorbate are present? c. How many moles of C are present? d. How many moles of Na are present? e. How many formula units of sodium ascorbate are present? f. How many atoms of Na are present? ...
... b. Consider a 500.-mg tablet. How many moles of sodium ascorbate are present? c. How many moles of C are present? d. How many moles of Na are present? e. How many formula units of sodium ascorbate are present? f. How many atoms of Na are present? ...
Polyamide from lactams by reactive rotational molding via anionic
... possible the synthesis of engineering thermoplastics, e.g., polyamide 6 (PA6), with high molecular weights. In the case of PA6, this is done through ...
... possible the synthesis of engineering thermoplastics, e.g., polyamide 6 (PA6), with high molecular weights. In the case of PA6, this is done through ...
Practice Problems in Biomedical Organic Chemistry
... This problem set was developed to assist undergraduate students taking a one semester or two semester, nonmajors course in organic chemistry. Students in these courses often come to organic chemistry from diverse backgrounds including biology, microbiology, and a variety of medical-related fields (e ...
... This problem set was developed to assist undergraduate students taking a one semester or two semester, nonmajors course in organic chemistry. Students in these courses often come to organic chemistry from diverse backgrounds including biology, microbiology, and a variety of medical-related fields (e ...
Lecture 13 11-20-02
... Since the analyte is diprotic there are two equivalence points, each requiring the same volume of titrant. Before the first equivalence point the pH is controlled by a buffer consisting of H 2A and HA-, and the HA-/A2- buffer determines the pH between the two equivalence points. After the second equ ...
... Since the analyte is diprotic there are two equivalence points, each requiring the same volume of titrant. Before the first equivalence point the pH is controlled by a buffer consisting of H 2A and HA-, and the HA-/A2- buffer determines the pH between the two equivalence points. After the second equ ...
WJEC Eduqas A Level Chemistry specification
... that learners must demonstrate a holistic understanding of the links between different areas of content. In practice this means that questions set in any component may require learners to draw upon knowledge from other parts of the specification. Each topic area includes an overview outlining the co ...
... that learners must demonstrate a holistic understanding of the links between different areas of content. In practice this means that questions set in any component may require learners to draw upon knowledge from other parts of the specification. Each topic area includes an overview outlining the co ...
GCE Chemistry SAMs 2009 onwards pdf
... Here are his results Mass of ethanol before experiment Mass of ethanol after experiment Mass of water Temperature of water before experiment Temperature of water after experiment ...
... Here are his results Mass of ethanol before experiment Mass of ethanol after experiment Mass of water Temperature of water before experiment Temperature of water after experiment ...
document
... The molar mass of Mg(OH)2 is 58.33 g so 1.00 g Mg(OH)2 x 1 mole / 58.33 g = 0.0171 mol ...
... The molar mass of Mg(OH)2 is 58.33 g so 1.00 g Mg(OH)2 x 1 mole / 58.33 g = 0.0171 mol ...
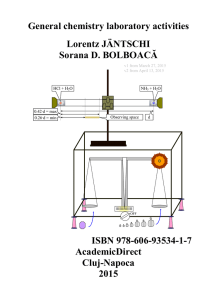
General chemistry laboratory activities, Lorentz
... Laboratory flasks are vessels (containers) which in laboratory and other scientific settings, they are usually referred to simply as flasks. Flasks come in a number of shapes and a wide range of sizes which are specified by the volume they can hold, typically in metric units such as millilitres (mL) ...
... Laboratory flasks are vessels (containers) which in laboratory and other scientific settings, they are usually referred to simply as flasks. Flasks come in a number of shapes and a wide range of sizes which are specified by the volume they can hold, typically in metric units such as millilitres (mL) ...
GCE Chemistry Specification (From 2015 - WALES ONLY
... draw together different areas of knowledge and understanding from across the full course of study. Each topic area includes an overview outlining the content and how it contributes to the wider aims of the specification. Knowledge of specific contexts and/or examples included in the overview will no ...
... draw together different areas of knowledge and understanding from across the full course of study. Each topic area includes an overview outlining the content and how it contributes to the wider aims of the specification. Knowledge of specific contexts and/or examples included in the overview will no ...
Chapter 15: Chemical Equilibrium
... When this equilibrium state is achieved, the concentrations of all the species in solution are constant, even though the forward and reverse reactions continue to take place. Note that when a system is at equilibrium, while the rates of the forward and reverse reactions are equal, the rate constants ...
... When this equilibrium state is achieved, the concentrations of all the species in solution are constant, even though the forward and reverse reactions continue to take place. Note that when a system is at equilibrium, while the rates of the forward and reverse reactions are equal, the rate constants ...
Quiz Keys - Section 10
... Name __________KEY_______________ Fall 2010 TuTh 5:00-6:15 pm, 206 BrL Quiz#7 Problem 1 (7 points). In class we discussed the problems associated with high altitude caused by lower boiling points of liquids at lower pressures. A different kind of problem may be caused by changing a boiling point of ...
... Name __________KEY_______________ Fall 2010 TuTh 5:00-6:15 pm, 206 BrL Quiz#7 Problem 1 (7 points). In class we discussed the problems associated with high altitude caused by lower boiling points of liquids at lower pressures. A different kind of problem may be caused by changing a boiling point of ...
msc_pre_chemistry_pap1_bl2
... for d6 octahedral complexes. At weak field (high spin complexes) the ground term is 5T2g, while at strong fields (low spin complexes) it is 1A1g Note that in the region immediately on each side of the spin crossover point, the energy difference between the two terms is small; thus high and low sp ...
... for d6 octahedral complexes. At weak field (high spin complexes) the ground term is 5T2g, while at strong fields (low spin complexes) it is 1A1g Note that in the region immediately on each side of the spin crossover point, the energy difference between the two terms is small; thus high and low sp ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.