Industrial Chemistry - Osun State University
... Energetic: Bond dissociation energies, Energy cycles including the Born-Haber cycle, applied to both covalent and ionic bonds. Limited accuracy of dissociation energies. Heats of formation and their determinations. Laws of thermodynamics. The concept of reversibility. The possibility of endothermic ...
... Energetic: Bond dissociation energies, Energy cycles including the Born-Haber cycle, applied to both covalent and ionic bonds. Limited accuracy of dissociation energies. Heats of formation and their determinations. Laws of thermodynamics. The concept of reversibility. The possibility of endothermic ...
Polymers
... A polymer that is obtained from only one type of monomer is known as homo polymer. On the otherhand polymer obtained from different type of monomers is referred to as copolymer. 2) Describe the classification of polymers? Polymers can be classified into several ways. (a).Based on Structure of Polyme ...
... A polymer that is obtained from only one type of monomer is known as homo polymer. On the otherhand polymer obtained from different type of monomers is referred to as copolymer. 2) Describe the classification of polymers? Polymers can be classified into several ways. (a).Based on Structure of Polyme ...
Modifying the stereochemistry of an enzyme
... here is a growing demand in the chemical and biotechnology industries for reliable and efficient methods for the production of enantiomerically pure compounds. Increasingly, synthetic chemists are exploiting enzymes in the asymmetric and stereoselective synthesis of chiral building blocks (1). Howev ...
... here is a growing demand in the chemical and biotechnology industries for reliable and efficient methods for the production of enantiomerically pure compounds. Increasingly, synthetic chemists are exploiting enzymes in the asymmetric and stereoselective synthesis of chiral building blocks (1). Howev ...
Slide 1
... the equilibrium to the left and reduces the concentration of hydrogen chloride. • Thus, the value of Keq decreases. • Lowering the temperature of the system means that heat is removed, so the equilibrium relieves the stress by shifting to the right, increasing both the concentration of hydrogen chlo ...
... the equilibrium to the left and reduces the concentration of hydrogen chloride. • Thus, the value of Keq decreases. • Lowering the temperature of the system means that heat is removed, so the equilibrium relieves the stress by shifting to the right, increasing both the concentration of hydrogen chlo ...
MC_16_mac
... • Thermochemical equations are usually written by designating a ∆H value rather than writing the energy as a reactant or product. • For an exothermic reaction, ∆H is negative because the system loses energy. • The thermochemical equation for the exothermic reaction previously discussed will look lik ...
... • Thermochemical equations are usually written by designating a ∆H value rather than writing the energy as a reactant or product. • For an exothermic reaction, ∆H is negative because the system loses energy. • The thermochemical equation for the exothermic reaction previously discussed will look lik ...
Packet 1 - Kentucky Community and Technical College System
... Because water is so abundant it is very useful to use as a solvent. The fact that so many ionic and molecular chemicals are soluble in it makes it even more useful. ...
... Because water is so abundant it is very useful to use as a solvent. The fact that so many ionic and molecular chemicals are soluble in it makes it even more useful. ...
J. Am. Chem. SOC. 1993,115, 7685-7695
... observed. The formation of the $-complex is readily indicated by the appearance of high-field resonances for the protons of the coordinated double bond of the arene at 6 3.213 (td, JH-H= JP-H = 7.4 Hz, JR&H = 2.5 Hz, as determined by IH-IH and 1H-31P decoupling experiments) and 3.629 (td, JH-H = JP- ...
... observed. The formation of the $-complex is readily indicated by the appearance of high-field resonances for the protons of the coordinated double bond of the arene at 6 3.213 (td, JH-H= JP-H = 7.4 Hz, JR&H = 2.5 Hz, as determined by IH-IH and 1H-31P decoupling experiments) and 3.629 (td, JH-H = JP- ...
Module 2
... thermodynamics. Entropy. Thermodynamic potentials: Gibbs’ free energy, Helmholtz’ free energy. Termodynamical equilibrium conditions. The criteria for the spontaneous processes direction. The basic principles of thermodynamics applying to living organisms. ATP as an energy source for biochemical rea ...
... thermodynamics. Entropy. Thermodynamic potentials: Gibbs’ free energy, Helmholtz’ free energy. Termodynamical equilibrium conditions. The criteria for the spontaneous processes direction. The basic principles of thermodynamics applying to living organisms. ATP as an energy source for biochemical rea ...
Acidic Environment
... Then the hydrogen ion reacts with water, ie this reaction occurs: H+ + H2O ...
... Then the hydrogen ion reacts with water, ie this reaction occurs: H+ + H2O ...
Contents - MCAT Prep Course
... The metal with the highest rise in temperature will be the metal with the lowest specific heat capacity. Specific heat capacity measures the amount of heat 1g of a substance can absorb before it rises in temperature by 1°C. Sr has the lowest specific heat capacity and therefore will rise in temperat ...
... The metal with the highest rise in temperature will be the metal with the lowest specific heat capacity. Specific heat capacity measures the amount of heat 1g of a substance can absorb before it rises in temperature by 1°C. Sr has the lowest specific heat capacity and therefore will rise in temperat ...
Enthalpy change - Don`t Trust Atoms
... • This is the temperature at which the reaction is just feasible. • In a closed system an equilibrium between products and reactants ...
... • This is the temperature at which the reaction is just feasible. • In a closed system an equilibrium between products and reactants ...
1 mol H 2
... at the end of a reaction is the same as was present at the beginning. The total mass of the reactants equals the mass of the products. ...
... at the end of a reaction is the same as was present at the beginning. The total mass of the reactants equals the mass of the products. ...
Document
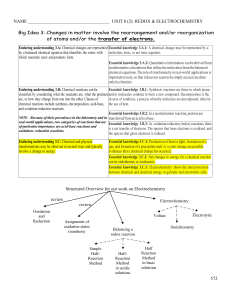
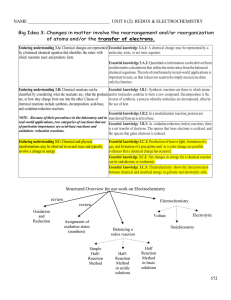
... chemical equations. The role of stoichiometry in real-world applications is important to note, so that it does not seem to be simply an exercise done only by chemists. Enduring understanding 3.B: Chemical reactions can be Essential knowledge 3.B.1: Synthesis reactions are those in which atoms classi ...
... chemical equations. The role of stoichiometry in real-world applications is important to note, so that it does not seem to be simply an exercise done only by chemists. Enduring understanding 3.B: Chemical reactions can be Essential knowledge 3.B.1: Synthesis reactions are those in which atoms classi ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.