Combustion and thermal degradation of polymers
... Photodegradation or light-induced polymer degradation, or, concerns the physical and chemical changes caused by irradiation of polymers with ultraviolet or visible light. In order to be effective, light must be absorbed by the substrate. Thus, the existence of chromophoric (light absorbing) groups ...
... Photodegradation or light-induced polymer degradation, or, concerns the physical and chemical changes caused by irradiation of polymers with ultraviolet or visible light. In order to be effective, light must be absorbed by the substrate. Thus, the existence of chromophoric (light absorbing) groups ...
Higher Chemistry Resources Guide - Glow Blogs
... dropping a strip of magnesium into various concentrations of hydrochloric acid and recording the time taken for the effervescence to stop. An unusual experiment demonstrating the effect of concentration on reaction rate is provided in the decolourisation of permanganate using rhubarb as described in ...
... dropping a strip of magnesium into various concentrations of hydrochloric acid and recording the time taken for the effervescence to stop. An unusual experiment demonstrating the effect of concentration on reaction rate is provided in the decolourisation of permanganate using rhubarb as described in ...
1 - A-Level Chemistry
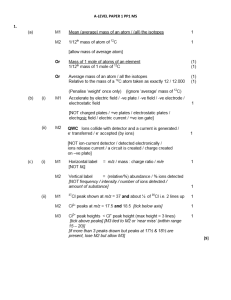
... no lone / spare / non-bonded pair of electrons only score M2 if M1 correct or give ‘H’ in M1 ...
... no lone / spare / non-bonded pair of electrons only score M2 if M1 correct or give ‘H’ in M1 ...
Higher Chemistry Resources Guide - Glow Blogs
... The following pages show the Mandatory Course key areas table from the SQA Higher Chemistry Course and Unit Support Notes. An additional fourth column has been included which contains hyperlinks to useful resources. Please note: Practitioners are not required to use the resources listed – they are o ...
... The following pages show the Mandatory Course key areas table from the SQA Higher Chemistry Course and Unit Support Notes. An additional fourth column has been included which contains hyperlinks to useful resources. Please note: Practitioners are not required to use the resources listed – they are o ...
Stoichiometry1
... position with each other. NaOH + HCl HOH + NaCl Fe(OH)2 + 2KBr 2KOH + FeBr2 Balancing an equation means that both sides must have the same number and type of atoms present. Coefficients are red (indicating total number of compounds), subscripts are blue (indicating number of atoms). ...
... position with each other. NaOH + HCl HOH + NaCl Fe(OH)2 + 2KBr 2KOH + FeBr2 Balancing an equation means that both sides must have the same number and type of atoms present. Coefficients are red (indicating total number of compounds), subscripts are blue (indicating number of atoms). ...
Problem 28. TUNNELING IN CHEMISTRY
... where Hvap = 30720 J/mol is the enthalpy of vaporization of benzene. Estimate the boiling point (T*) of the finely dispersed liquid benzene at the standard atmospheric pressure if the sample consists of droplets with the radius r = 50 nm. The surface tension of benzene is 0.029 J/m2 and its density ...
... where Hvap = 30720 J/mol is the enthalpy of vaporization of benzene. Estimate the boiling point (T*) of the finely dispersed liquid benzene at the standard atmospheric pressure if the sample consists of droplets with the radius r = 50 nm. The surface tension of benzene is 0.029 J/m2 and its density ...
5 organic chemistry: functional groups
... The longest chain contains the OOH group, which means the compound is named as a derivative of octane. Because it is an alcohol, it would be tempting to name it as an octanol. But it contains a CPC double bond, which means it must be an octenol. We now have to indicate that the OOH group is on one e ...
... The longest chain contains the OOH group, which means the compound is named as a derivative of octane. Because it is an alcohol, it would be tempting to name it as an octanol. But it contains a CPC double bond, which means it must be an octenol. We now have to indicate that the OOH group is on one e ...
Equilibrium - chemmybear.com
... At elevated temperatures, SbCl5 gas decomposes into SbCl3 gas and Cl2 gas as shown by the following equation: SbCl5(g) SbCl3(g) + Cl2(g) (a) An 89.7 gram sample of SbCl5 (molecular weight 299.0) is placed in an evacuated 15.0 litre container at 182ºC. 1. What is the concentration in moles per litr ...
... At elevated temperatures, SbCl5 gas decomposes into SbCl3 gas and Cl2 gas as shown by the following equation: SbCl5(g) SbCl3(g) + Cl2(g) (a) An 89.7 gram sample of SbCl5 (molecular weight 299.0) is placed in an evacuated 15.0 litre container at 182ºC. 1. What is the concentration in moles per litr ...
AP Chem unit 13 presentation
... to the reaction may alter the equilibrium positions, they do not alter the equilibrium constant. ...
... to the reaction may alter the equilibrium positions, they do not alter the equilibrium constant. ...
Document
... The excess reagent is the one you have left over. The limiting reagent determines how much product you can make ...
... The excess reagent is the one you have left over. The limiting reagent determines how much product you can make ...
Review Unit: Chemistry Review
... or dangerous consequences. In fact, the comfortable lives we lead are due in large part to our understanding and application of chemistry. Some chemicals are harmful to people or the environment, but many are integral to life, such as the carbon dioxide, oxygen, water, and glucose in the cycle of ph ...
... or dangerous consequences. In fact, the comfortable lives we lead are due in large part to our understanding and application of chemistry. Some chemicals are harmful to people or the environment, but many are integral to life, such as the carbon dioxide, oxygen, water, and glucose in the cycle of ph ...
Chapter 4
... microscopic level, that is, whether the solute particles are ions, molecules, or a mixture of the two. Nevertheless, some of the earliest insights into the microscopic nature of aqueous solutions came through macroscopic observations on the ability of solutions to conduct electricity. To understand ...
... microscopic level, that is, whether the solute particles are ions, molecules, or a mixture of the two. Nevertheless, some of the earliest insights into the microscopic nature of aqueous solutions came through macroscopic observations on the ability of solutions to conduct electricity. To understand ...
4U Chemistry Practice Exam - Coristines
... b. Amines are non-polar molecules. c. Amines always have a larger molecular weight than amides. d. Amines always have a nitrogen atom attached to two carbon atoms. e. Amines can be found in proteins, but amides can not. 5. Why does the boiling point of an alkane increase as its chain length increase ...
... b. Amines are non-polar molecules. c. Amines always have a larger molecular weight than amides. d. Amines always have a nitrogen atom attached to two carbon atoms. e. Amines can be found in proteins, but amides can not. 5. Why does the boiling point of an alkane increase as its chain length increase ...
Theoretical studies of systems of biochemical interest containing Fe
... The understanding of the origin of the way to reduce ΔG‡ requires finding out how the enzymes can stabilize their transition state more than the transition state of the uncatalyzed reaction. The exact strategy they adopt to accelerate reaction rates by a factor as large as 1017 is a very interesting ...
... The understanding of the origin of the way to reduce ΔG‡ requires finding out how the enzymes can stabilize their transition state more than the transition state of the uncatalyzed reaction. The exact strategy they adopt to accelerate reaction rates by a factor as large as 1017 is a very interesting ...
Oxidation numbers
... Hydrogen will have an oxidation number of +1 (rule 6, ammonia is not a metal hydride). At this stage we do not know the oxidation number for nitrogen. Determine the oxidation number of nitrogen by using the fact that the oxidation numbers of the atoms must add up to the charge on the compound In the ...
... Hydrogen will have an oxidation number of +1 (rule 6, ammonia is not a metal hydride). At this stage we do not know the oxidation number for nitrogen. Determine the oxidation number of nitrogen by using the fact that the oxidation numbers of the atoms must add up to the charge on the compound In the ...
Ch. 18 Class PowerPoint
... • But theoretically, every reaction can proceed in two directions, forward and reverse. • Essentially all chemical reactions are considered to be reversible under suitable conditions. • A chemical reaction in which the products can react to re-form the reactants is called a reversible reaction. ...
... • But theoretically, every reaction can proceed in two directions, forward and reverse. • Essentially all chemical reactions are considered to be reversible under suitable conditions. • A chemical reaction in which the products can react to re-form the reactants is called a reversible reaction. ...
Determination of Equilibrium Constants for the
... of the in-plane ring HBMC, but state that it does not connect to the transition state that leads to 2-HIPP. It is not clear that this connectivity issue makes a significant impact, since at the internal energies needed to surmount the TS barrier in their work, the two isomers are likely to interconve ...
... of the in-plane ring HBMC, but state that it does not connect to the transition state that leads to 2-HIPP. It is not clear that this connectivity issue makes a significant impact, since at the internal energies needed to surmount the TS barrier in their work, the two isomers are likely to interconve ...
here
... hypothesis that maggots are created from rotting meat. Once a hypothesis has been formulated, scientists test it with more rigorous experiments. For example, after forming the hypothesis that maggots are created from rotting meat, early scientists did experiments to make sure that the maggots were n ...
... hypothesis that maggots are created from rotting meat. Once a hypothesis has been formulated, scientists test it with more rigorous experiments. For example, after forming the hypothesis that maggots are created from rotting meat, early scientists did experiments to make sure that the maggots were n ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.