Week 8 – Intermolecular Forces
... (C) The bond length of Br2 is longer as opposed to the shorter bond lengths of Cl2. (D) Br2 molecules have electron clouds that are more polarizable than those of Cl2 molecules, therefore the London dispersion forces are stronger in liquid Br2. (E) Cl2 has a greater electronegativity than Br2, there ...
... (C) The bond length of Br2 is longer as opposed to the shorter bond lengths of Cl2. (D) Br2 molecules have electron clouds that are more polarizable than those of Cl2 molecules, therefore the London dispersion forces are stronger in liquid Br2. (E) Cl2 has a greater electronegativity than Br2, there ...
Electronic Structure and the Periodic Table A. Bohr Model of the
... A. Bohr Model of the Atom 1. Solar System Model 2. Created to Fit a “Quantized” Picture of Energy Transfer 3. Basis: Noncontinuous Emission Spectra of the Elements 4. Basic Postulates a. Electrons reside in certain allowed energy states b. Energy absorption and emission by atoms is caused by electro ...
... A. Bohr Model of the Atom 1. Solar System Model 2. Created to Fit a “Quantized” Picture of Energy Transfer 3. Basis: Noncontinuous Emission Spectra of the Elements 4. Basic Postulates a. Electrons reside in certain allowed energy states b. Energy absorption and emission by atoms is caused by electro ...
ppt
... VB theory assumes that the electrons are localized between the two bonding atoms Molecular orbital theory: electrons are spread throughout the entire molecule; electrons are delocalized over the whole molecule. Pure atomic orbitals combine to produce molecular orbitals that are spread out, delocali ...
... VB theory assumes that the electrons are localized between the two bonding atoms Molecular orbital theory: electrons are spread throughout the entire molecule; electrons are delocalized over the whole molecule. Pure atomic orbitals combine to produce molecular orbitals that are spread out, delocali ...
PDF (6)
... • oxidation number = valence electrons – nonbonding electrons – ‘assigned’ bonding electrons† † bonding electrons ‘assigned’ to more electronegative atom in bond ...
... • oxidation number = valence electrons – nonbonding electrons – ‘assigned’ bonding electrons† † bonding electrons ‘assigned’ to more electronegative atom in bond ...
Chapter Outline • Review of Atomic Structure Electrons, protons
... As a result of this transfer mutual ionization occurs: atom that gives up electron(s) becomes positively charged ion (cation), atom that accepts electron(s) becomes negatively charged ion (anion). Formation of ionic bond: 1. Mutual ionization occurs by electron transfer (remember electronegativity t ...
... As a result of this transfer mutual ionization occurs: atom that gives up electron(s) becomes positively charged ion (cation), atom that accepts electron(s) becomes negatively charged ion (anion). Formation of ionic bond: 1. Mutual ionization occurs by electron transfer (remember electronegativity t ...
2A Final Exam Review Worksheet
... A. If there is 10.0 g of P4O10, find the mass of phosphoric acid formed. B. If there is also 10.0 g of perchloric acid, find the mass of phosphoric acid formed. C. Considering A & B, how much of the excess reactant remains after the reaction is complete. D. Find the number of phosphorus atoms in 10. ...
... A. If there is 10.0 g of P4O10, find the mass of phosphoric acid formed. B. If there is also 10.0 g of perchloric acid, find the mass of phosphoric acid formed. C. Considering A & B, how much of the excess reactant remains after the reaction is complete. D. Find the number of phosphorus atoms in 10. ...
21Sc , 48 22Ti , 50 22Ti , 50
... as bromine-79 (78.9183 u), what is the mass of the other isotope? 8. You have 0.330 mole of each of the following elements: Be, B, Br, Ba, and Bi. Which sample has the largest mass? 9. A 3.6 g sample of lithium contains ________ atoms. 10. How many elements are in the fifth period of the periodic ta ...
... as bromine-79 (78.9183 u), what is the mass of the other isotope? 8. You have 0.330 mole of each of the following elements: Be, B, Br, Ba, and Bi. Which sample has the largest mass? 9. A 3.6 g sample of lithium contains ________ atoms. 10. How many elements are in the fifth period of the periodic ta ...
Define:
... 44. Express the sum of 8.67 m and 5.2 m to the correct number of significant figures. 45. Express the product of 5.5 mm and 2.00 mm to the correct number of significant figures. 46. List the metric prefixes and their decimal equivalents. Ex: centi .01 47. Make the following conversions: a. 8961 m to ...
... 44. Express the sum of 8.67 m and 5.2 m to the correct number of significant figures. 45. Express the product of 5.5 mm and 2.00 mm to the correct number of significant figures. 46. List the metric prefixes and their decimal equivalents. Ex: centi .01 47. Make the following conversions: a. 8961 m to ...
2·QUIZLET VOCABULARY: Quantum Numbers Study online at
... 3. electron configuration: the arrangement of electrons around nucleus in an atom 4. Hunds rule: orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin 5. Magnetic (orbital) ...
... 3. electron configuration: the arrangement of electrons around nucleus in an atom 4. Hunds rule: orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin 5. Magnetic (orbital) ...
AP Biology
... Web/CD Activity2B: Structure of the Atomic Nucleus Web/CD Activity2C: Electron Arrangement Web/CD Activity2D: Build an Atom Atoms combine by chemical bonding to form molecules (pp. 33-36, FIGURES 2.12 and 2.14) Chemical bonds form when atoms interact and complete their valence shells. A covalent b ...
... Web/CD Activity2B: Structure of the Atomic Nucleus Web/CD Activity2C: Electron Arrangement Web/CD Activity2D: Build an Atom Atoms combine by chemical bonding to form molecules (pp. 33-36, FIGURES 2.12 and 2.14) Chemical bonds form when atoms interact and complete their valence shells. A covalent b ...
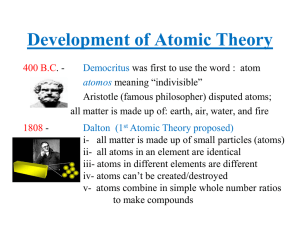
Development of Atomic Theory
... Schrodinger’s model of the atom is just a more in depth approach to Bohr’s model, but involves mathematically derived differential equations. ...
... Schrodinger’s model of the atom is just a more in depth approach to Bohr’s model, but involves mathematically derived differential equations. ...
72KB
... Graphite consists of C atoms each covalently bonded to three other C atoms in a 2-D or layered arrangement with weak intermolecular forces of attraction between the layers or sheets. In diamond, the covalent bonds between the carbon atoms are very strong and hold the atoms in place, making it diffic ...
... Graphite consists of C atoms each covalently bonded to three other C atoms in a 2-D or layered arrangement with weak intermolecular forces of attraction between the layers or sheets. In diamond, the covalent bonds between the carbon atoms are very strong and hold the atoms in place, making it diffic ...
02-Atoms-Molecules
... Reactive elements do not have energy level fully occupied by electrons their outermost energy level fully ...
... Reactive elements do not have energy level fully occupied by electrons their outermost energy level fully ...
Atomic Theory - Fort Thomas Independent Schools
... Theory of Matter originally only gasses. • As pointed out by Albert Einstein in 1905, this experimental evidence for kinetic theory is generally seen as having confirmed the existence of atoms and molecules. ...
... Theory of Matter originally only gasses. • As pointed out by Albert Einstein in 1905, this experimental evidence for kinetic theory is generally seen as having confirmed the existence of atoms and molecules. ...
Define:
... 43. Express the sum of 8.67 m and 5.2 m to the correct number of significant figures. 44. Express the product of 5.5 mm and 2.00 mm to the correct number of significant figures. 45. List the metric prefixes and their decimal equivalents. Ex: centi .01 46. Make the following conversions: a. 8961 m to ...
... 43. Express the sum of 8.67 m and 5.2 m to the correct number of significant figures. 44. Express the product of 5.5 mm and 2.00 mm to the correct number of significant figures. 45. List the metric prefixes and their decimal equivalents. Ex: centi .01 46. Make the following conversions: a. 8961 m to ...
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electrostatic force of attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction. The strength of chemical bonds varies considerably; there are ""strong bonds"" such as covalent or ionic bonds and ""weak bonds"" such as Dipole-dipole interaction, the London dispersion force and hydrogen bonding.Since opposite charges attract via a simple electromagnetic force, the negatively charged electrons that are orbiting the nucleus and the positively charged protons in the nucleus attract each other. An electron positioned between two nuclei will be attracted to both of them, and the nuclei will be attracted toward electrons in this position. This attraction constitutes the chemical bond. Due to the matter wave nature of electrons and their smaller mass, they must occupy a much larger amount of volume compared with the nuclei, and this volume occupied by the electrons keeps the atomic nuclei relatively far apart, as compared with the size of the nuclei themselves. This phenomenon limits the distance between nuclei and atoms in a bond.In general, strong chemical bonding is associated with the sharing or transfer of electrons between the participating atoms. The atoms in molecules, crystals, metals and diatomic gases—indeed most of the physical environment around us—are held together by chemical bonds, which dictate the structure and the bulk properties of matter.All bonds can be explained by quantum theory, but, in practice, simplification rules allow chemists to predict the strength, directionality, and polarity of bonds. The octet rule and VSEPR theory are two examples. More sophisticated theories are valence bond theory which includes orbital hybridization and resonance, and the linear combination of atomic orbitals molecular orbital method which includes ligand field theory. Electrostatics are used to describe bond polarities and the effects they have on chemical substances.