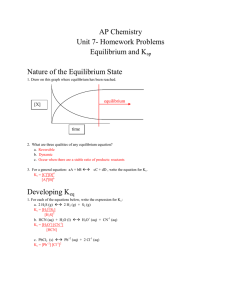
AP Chemistry Unit 7- Homework Problems Equilibrium and Ksp
... AuCl (Ksp = 2x10-13) MnCO3 (Ksp = 2.3x10-11) PbCrO4 (Ksp = 2.8x10-13) Since they are all the same number of pieces, you can compare them directly by K sp values. Thus: AuCl (Ksp = 2x10-13) < PbCrO4 (Ksp = 2.8x10-13) < MnCO3 (Ksp = 2.3x10-11) < NiCO3 (Ksp = 1.4x10-7) 10. Put the following substances ...
... AuCl (Ksp = 2x10-13) MnCO3 (Ksp = 2.3x10-11) PbCrO4 (Ksp = 2.8x10-13) Since they are all the same number of pieces, you can compare them directly by K sp values. Thus: AuCl (Ksp = 2x10-13) < PbCrO4 (Ksp = 2.8x10-13) < MnCO3 (Ksp = 2.3x10-11) < NiCO3 (Ksp = 1.4x10-7) 10. Put the following substances ...
CHM 4XX. Organometallic Chemistry (0.5
... end we have created a new major program which gives students options in choosing courses. Similarly the new minor program also introduces flexibility not currently possible. These revisions will allow students to tailor a curriculum as appropriate for their interests. They also offer more flexibilit ...
... end we have created a new major program which gives students options in choosing courses. Similarly the new minor program also introduces flexibility not currently possible. These revisions will allow students to tailor a curriculum as appropriate for their interests. They also offer more flexibilit ...
Chemistry (Revised)
... FeS(s) + 2HCl(aq) → FeCl2(aq) + H2S(g) (i) Hydrogen sulfide gas is very soluble in water. Draw a diagram to show an assembled apparatus that could be used to measure the volume of H2S gas produced when a sample of ...
... FeS(s) + 2HCl(aq) → FeCl2(aq) + H2S(g) (i) Hydrogen sulfide gas is very soluble in water. Draw a diagram to show an assembled apparatus that could be used to measure the volume of H2S gas produced when a sample of ...
Experimental Chemistry I
... considered as a neutralization reaction. In a neutralization reaction, an acid reacts with a base to produce salt and water: NaOH(aq) + HCl(aq) → 2H2O(l) + Na+(aq) + Cl-(aq) An indicator enables detection of the stoichiometric point (S), the stage at which the volume of titrant added (with a given c ...
... considered as a neutralization reaction. In a neutralization reaction, an acid reacts with a base to produce salt and water: NaOH(aq) + HCl(aq) → 2H2O(l) + Na+(aq) + Cl-(aq) An indicator enables detection of the stoichiometric point (S), the stage at which the volume of titrant added (with a given c ...
kcse chemistry questions
... What condition is necessary for the process in step I to take place? (1mk) Draw a labeled diagram for the set-up that could be used to separate the mixture formed in step II (2mks) Write ionic equation for the reaction between the cation in filtrate X and aqueous ammonia. (1mk) What observation woul ...
... What condition is necessary for the process in step I to take place? (1mk) Draw a labeled diagram for the set-up that could be used to separate the mixture formed in step II (2mks) Write ionic equation for the reaction between the cation in filtrate X and aqueous ammonia. (1mk) What observation woul ...
D--All Websites-eChemistryHelp-.mdi
... (ii) Oxidation Number of Cl in ClF3 = +3 (iii) Oxidation Number of Cl in KClO3 = +5 (iv) Oxidation Number of I in IF7 = +7 (v) Oxidation Number of I in IF5 = +5 Oxidation Number of Radicals Oxidation Number of radicals is equal to charge present on them. For example, (i) Oxidation Number of sulphite ...
... (ii) Oxidation Number of Cl in ClF3 = +3 (iii) Oxidation Number of Cl in KClO3 = +5 (iv) Oxidation Number of I in IF7 = +7 (v) Oxidation Number of I in IF5 = +5 Oxidation Number of Radicals Oxidation Number of radicals is equal to charge present on them. For example, (i) Oxidation Number of sulphite ...
Chapter 18 pdf
... When the stopcock in the tube connecting the two flasks is opened, iodine vapor can travel back and forth between the two flasks. After a period of time, the readings on the Geiger counters indicate that the flask on the left contains as many radioactive I-131 molecules as the flask on the right in ...
... When the stopcock in the tube connecting the two flasks is opened, iodine vapor can travel back and forth between the two flasks. After a period of time, the readings on the Geiger counters indicate that the flask on the left contains as many radioactive I-131 molecules as the flask on the right in ...
Instructor`s Resource Manual
... General Chemistry, Eighth Edition, is designed to give the instructor the greatest flexibility in creating a course for his or her students and to make the process of teaching with the text as smooth as possible. The careful, logical, and clear development of material in each chapter, with its appro ...
... General Chemistry, Eighth Edition, is designed to give the instructor the greatest flexibility in creating a course for his or her students and to make the process of teaching with the text as smooth as possible. The careful, logical, and clear development of material in each chapter, with its appro ...
Chapter 4: Reactions in Aqueous Solution
... A) H2O B) CH3OH C) CH3CH2OH D) HF E) NaF Ans: E Category: Easy Section: 4.1 2. Which of the following compounds is a weak electrolyte? A) HNO3 B) NaNO3 C) HNO2 D) NaNO2 E) NaOH Ans: C Category: Easy Section: 4.1 3. Which of the following compounds is a strong electrolyte? A) H2O D) CH3CH2OH (ethanol ...
... A) H2O B) CH3OH C) CH3CH2OH D) HF E) NaF Ans: E Category: Easy Section: 4.1 2. Which of the following compounds is a weak electrolyte? A) HNO3 B) NaNO3 C) HNO2 D) NaNO2 E) NaOH Ans: C Category: Easy Section: 4.1 3. Which of the following compounds is a strong electrolyte? A) H2O D) CH3CH2OH (ethanol ...
Solutions Manual
... Cellulose is made from repeating units of β-glucose with inversion of every second unit. This produces long, straight chains of cellulose which are linked to each other by hydrogen bonding. In plants, cellulose acts as a structural material. Starch (both amylase and amylopectin) is made from long-ch ...
... Cellulose is made from repeating units of β-glucose with inversion of every second unit. This produces long, straight chains of cellulose which are linked to each other by hydrogen bonding. In plants, cellulose acts as a structural material. Starch (both amylase and amylopectin) is made from long-ch ...
HOTS Worksheet
... Ans. The (— CO — NH —) amide bond in nylon gets hydrolysed. Q. 2. Fibres are of crystalline structure. Why ? Ans. Fibres have strong intermolecular forces of attraction which leads to close packing of their chains and impart crystalline structure. Q. 3. Which artificial polymer is present in bubble ...
... Ans. The (— CO — NH —) amide bond in nylon gets hydrolysed. Q. 2. Fibres are of crystalline structure. Why ? Ans. Fibres have strong intermolecular forces of attraction which leads to close packing of their chains and impart crystalline structure. Q. 3. Which artificial polymer is present in bubble ...
Document
... irradiated with electromagnetic radiations, are known as intrinsic semiconductor. This happens because certain covalent bonds are broken and the released electrons are in a position to conduct electric current. e.g. Silicon , Germanium. ...
... irradiated with electromagnetic radiations, are known as intrinsic semiconductor. This happens because certain covalent bonds are broken and the released electrons are in a position to conduct electric current. e.g. Silicon , Germanium. ...
REACTIONS IN AQUEOUS SOLUTION
... precipitation reactions. A precipitate is an insoluble solid formed by a reaction in solution. In Figure 4.4 the precipitate is lead iodide (PbI2), a compound that has a very low solubility in water: ...
... precipitation reactions. A precipitate is an insoluble solid formed by a reaction in solution. In Figure 4.4 the precipitate is lead iodide (PbI2), a compound that has a very low solubility in water: ...
Disproportionation of Gold(II)
... showed this conclusion could be extended to neutral, threecoordinate AuI complexes of the type Au(phosphine)2X (X ) univalent anion, e.g., chloride, bromide or iodide). The results of these theoretical predictions have been utilized by the experimental group of Omary to design series of novel lumine ...
... showed this conclusion could be extended to neutral, threecoordinate AuI complexes of the type Au(phosphine)2X (X ) univalent anion, e.g., chloride, bromide or iodide). The results of these theoretical predictions have been utilized by the experimental group of Omary to design series of novel lumine ...
mclintock.ch6 [Compatibility Mode]
... ► Acid–base neutralization reactions are processes in which H+ ions from an acid react with OH- ions from a base to yield water. An ionic compound called a salt is also produced. The “salt” produced need not be common table salt. Any ionic compound produced in an acid–base reaction is called a salt. ...
... ► Acid–base neutralization reactions are processes in which H+ ions from an acid react with OH- ions from a base to yield water. An ionic compound called a salt is also produced. The “salt” produced need not be common table salt. Any ionic compound produced in an acid–base reaction is called a salt. ...
Answers
... the empirical formula, which shows only the simplest whole number ratio of one atom to another. It conveys the least information about a molecule. ...
... the empirical formula, which shows only the simplest whole number ratio of one atom to another. It conveys the least information about a molecule. ...
CHAPTER 9
... of the scientific revolution, very little was known about the process of combustion. In attempting to explain this common phenomenon, chemists of the 18th century developed one of the first universally accepted theories in their field. But as one man would show, scientific theories do not always sta ...
... of the scientific revolution, very little was known about the process of combustion. In attempting to explain this common phenomenon, chemists of the 18th century developed one of the first universally accepted theories in their field. But as one man would show, scientific theories do not always sta ...

















![mclintock.ch6 [Compatibility Mode]](http://s1.studyres.com/store/data/003971396_1-780a12aa3165c9221aca3ac594a06674-300x300.png)


