sch103manual - university of nairobi staff profiles
... Under certain conditions of pressure and temperature, most substances can exist in any of the three states of matter: Solids, liquid or gas. Water for example, exists in the solid state as ice, liquid state as water and in the gaseous state as steam. The physical properties of a substance often depe ...
... Under certain conditions of pressure and temperature, most substances can exist in any of the three states of matter: Solids, liquid or gas. Water for example, exists in the solid state as ice, liquid state as water and in the gaseous state as steam. The physical properties of a substance often depe ...
VOLUME 3 - ICHO 41-45 _opravené_
... Method A – from X-ray diffraction data (modern) The unit cell is the smallest repeating unit in a crystal structure. The unit cell of a gold crystal is found by X-ray diffraction to have the face-centred cubic unit structure (i.e. where the centre of an atom is located at each corner of a cube and i ...
... Method A – from X-ray diffraction data (modern) The unit cell is the smallest repeating unit in a crystal structure. The unit cell of a gold crystal is found by X-ray diffraction to have the face-centred cubic unit structure (i.e. where the centre of an atom is located at each corner of a cube and i ...
Chapter 4 "Reactions in Aqueous Solution"
... information on periodic table groups and covalent bonding, see Chapter 2 "Molecules, Ions, and Chemical Formulas" and Chapter 7 "The Periodic Table and Periodic Trends".) Consequently, the oxygen and hydrogen nuclei do not equally share electrons. Instead, hydrogen atoms are electron poor compared w ...
... information on periodic table groups and covalent bonding, see Chapter 2 "Molecules, Ions, and Chemical Formulas" and Chapter 7 "The Periodic Table and Periodic Trends".) Consequently, the oxygen and hydrogen nuclei do not equally share electrons. Instead, hydrogen atoms are electron poor compared w ...
Reactions in Aqueous Solution
... information on periodic table groups and covalent bonding, see Chapter 2 "Molecules, Ions, and Chemical Formulas" and Chapter 7 "The Periodic Table and Periodic Trends".) Consequently, the oxygen and hydrogen nuclei do not equally share electrons. Instead, hydrogen atoms are electron poor compared w ...
... information on periodic table groups and covalent bonding, see Chapter 2 "Molecules, Ions, and Chemical Formulas" and Chapter 7 "The Periodic Table and Periodic Trends".) Consequently, the oxygen and hydrogen nuclei do not equally share electrons. Instead, hydrogen atoms are electron poor compared w ...
Mole-mole factor
... • Reasons include: • Increase the rate of reaction • To ensure that one reactant is completely used up (reacted) • Reactants are not completely converted to products as stated on paper (theory) ...
... • Reasons include: • Increase the rate of reaction • To ensure that one reactant is completely used up (reacted) • Reactants are not completely converted to products as stated on paper (theory) ...
Chapter 9 Stoichiometry
... Review: The Mole The number equal to the number of carbon atoms in exactly 12 grams of pure 12C. 1 mole of anything = 6.022 ´ 1023 units of that thing ...
... Review: The Mole The number equal to the number of carbon atoms in exactly 12 grams of pure 12C. 1 mole of anything = 6.022 ´ 1023 units of that thing ...
Chapter Four - Salina USD 305
... • If a reaction indicates that a catalyst was present, it is not part of the actual reaction. Instead, you write the catalyst above the arrow. • Catalysts are not used up in the reaction. They are just there to speed it up. • If a reaction is heated, you draw a triangle above the arrow. Heat is used ...
... • If a reaction indicates that a catalyst was present, it is not part of the actual reaction. Instead, you write the catalyst above the arrow. • Catalysts are not used up in the reaction. They are just there to speed it up. • If a reaction is heated, you draw a triangle above the arrow. Heat is used ...
chemistry - Textbooks Online
... Chemistry, a branch of science concerned with the properties, structures and composition of substances and their reactions with one another. Inorganic Chemistry studies the preparation, properties and reactions of all chemical elements and their compounds, except those of carbon. Organic Chemistry s ...
... Chemistry, a branch of science concerned with the properties, structures and composition of substances and their reactions with one another. Inorganic Chemistry studies the preparation, properties and reactions of all chemical elements and their compounds, except those of carbon. Organic Chemistry s ...
CHAPTER 9 Stoichiometry - Modern Chemistry Textbook
... T he chemical equation plays a very important part in all stoichiometric calculations because the mole ratio is obtained directly from it. Solving any reaction-stoichiometry problem must begin with a balanced equation. Chemical equations help us make predictions about chemical reactions without havi ...
... T he chemical equation plays a very important part in all stoichiometric calculations because the mole ratio is obtained directly from it. Solving any reaction-stoichiometry problem must begin with a balanced equation. Chemical equations help us make predictions about chemical reactions without havi ...
THE SYNTHESIS AND CHARACTERIZATION OF PHOSPHONIUM INDENYLIDE COMPLEXES OF RUTHENIUM(II)
... Figure 10. Transition metal complexes of phosphonium cyclopentadienylides which have been charactereized by X-ray crystallography (excludes complexes of group 8 metals) ........................ 9 Figure 11. Synthesis of (C5H4PPh3)M(CO)3 (M = Cr, Mo, W) ............................................... ...
... Figure 10. Transition metal complexes of phosphonium cyclopentadienylides which have been charactereized by X-ray crystallography (excludes complexes of group 8 metals) ........................ 9 Figure 11. Synthesis of (C5H4PPh3)M(CO)3 (M = Cr, Mo, W) ............................................... ...
Photo-oxidation of pinonaldehyde at low NOx
... aerosol (SOA) mass yields. Under concurrent UV illumination, mass yields are lower than high-NOx yields published earlier by our group. However, when OH radicals are produced via dark ozonolysis the SOA mass yields are comparable at high and low NOx . Because pinonaldehyde is a major first-generatio ...
... aerosol (SOA) mass yields. Under concurrent UV illumination, mass yields are lower than high-NOx yields published earlier by our group. However, when OH radicals are produced via dark ozonolysis the SOA mass yields are comparable at high and low NOx . Because pinonaldehyde is a major first-generatio ...
AP Chemistry - Siva Kodali
... degree in biochemistry and molecular biology from the University of Wisconsin–Eau Claire, and a PhD in biological chemistry from Indiana University. With science seething in his DNA, he sought to infect others with a sense of molecular wonderment. Having taught, tutored, and mentored in classroom an ...
... degree in biochemistry and molecular biology from the University of Wisconsin–Eau Claire, and a PhD in biological chemistry from Indiana University. With science seething in his DNA, he sought to infect others with a sense of molecular wonderment. Having taught, tutored, and mentored in classroom an ...
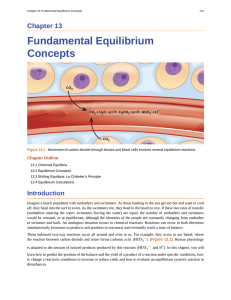
Fundamental Equilibrium Concepts
... The formation of NO2 from N2O4 is a reversible reaction, which is identified by the equilibrium arrow (⇌) . All reactions are reversible, but many reactions, for all practical purposes, proceed in one direction until the reactants are exhausted and will reverse only under certain conditions. Such re ...
... The formation of NO2 from N2O4 is a reversible reaction, which is identified by the equilibrium arrow (⇌) . All reactions are reversible, but many reactions, for all practical purposes, proceed in one direction until the reactants are exhausted and will reverse only under certain conditions. Such re ...
Chapter 3 Stoichiometry: Calculations with Chemical
... • One mole of atoms, ions, or molecules contains Avogadro’s number of those particles • One mole of molecules or formula units contains Avogadro’s number times the number of atoms or ions of each element in the compound Stoichiometry ...
... • One mole of atoms, ions, or molecules contains Avogadro’s number of those particles • One mole of molecules or formula units contains Avogadro’s number times the number of atoms or ions of each element in the compound Stoichiometry ...
Calculations with Chemical Formulas and Equations
... • One mole of atoms, ions, or molecules contains Avogadro’s number of those particles • One mole of molecules or formula units contains Avogadro’s number times the number of atoms or ions of each element in the compound Stoichiometry ...
... • One mole of atoms, ions, or molecules contains Avogadro’s number of those particles • One mole of molecules or formula units contains Avogadro’s number times the number of atoms or ions of each element in the compound Stoichiometry ...
Chapter 3 Stoichiometry: Calculations with Chemical Formulas and
... • One mole of atoms, ions, or molecules contains Avogadro s number of those particles • One mole of molecules or formula units contains Avogadro s number times the number of atoms or ions of each element in the compound Stoichiometry ...
... • One mole of atoms, ions, or molecules contains Avogadro s number of those particles • One mole of molecules or formula units contains Avogadro s number times the number of atoms or ions of each element in the compound Stoichiometry ...
Section – B - About iTutoring
... (2). What is called chemical equilibrium? The equilibrium established in chemical reactions is called chemical equilibrium. (3). How can be said that equilibrium is dynamic in nature? The equilibrium is dynamic and not steady as the forward and the reverse reactions occur with the same velocity at t ...
... (2). What is called chemical equilibrium? The equilibrium established in chemical reactions is called chemical equilibrium. (3). How can be said that equilibrium is dynamic in nature? The equilibrium is dynamic and not steady as the forward and the reverse reactions occur with the same velocity at t ...
purdue university - IUPUI ScholarWorks
... Three parts consist of my thesis work centered on the synthesis of inorganic phosphates and then metal organic frame work (MOF). The first part is the synthesis of mesoporous chromium phosphates using the room temperature solid state reaction (SSR) approach. One of the major aims of this work is to ...
... Three parts consist of my thesis work centered on the synthesis of inorganic phosphates and then metal organic frame work (MOF). The first part is the synthesis of mesoporous chromium phosphates using the room temperature solid state reaction (SSR) approach. One of the major aims of this work is to ...
Chemistry - SSA Punjab
... Calculate the temp. of 5.5 moles of a gas occupying 6 dm3 at 3.35 bar (R=0.083 bar dm3 k-1 mol-1) ...
... Calculate the temp. of 5.5 moles of a gas occupying 6 dm3 at 3.35 bar (R=0.083 bar dm3 k-1 mol-1) ...
Crosslinking Technical Handbook
... Crosslinking is the process of chemically joining two or more molecules by a covalent bond. Crosslinking reagents contain reactive ends to specific functional groups (primary amines, sulfhydryls, etc.) on proteins or other molecules. The availability of several chemical groups in proteins and peptid ...
... Crosslinking is the process of chemically joining two or more molecules by a covalent bond. Crosslinking reagents contain reactive ends to specific functional groups (primary amines, sulfhydryls, etc.) on proteins or other molecules. The availability of several chemical groups in proteins and peptid ...
Synthetic Organic Chemistry - Name
... reagent the extent of addition is reduced or the reaction not take place or some abnormal product is formed. If Grignard reagent has β-hydrogen atom, then in some hindred ketone also show reduction of carbonyl group , may be due to β-hydrogen transfer by the formation of six membered transition stat ...
... reagent the extent of addition is reduced or the reaction not take place or some abnormal product is formed. If Grignard reagent has β-hydrogen atom, then in some hindred ketone also show reduction of carbonyl group , may be due to β-hydrogen transfer by the formation of six membered transition stat ...









![1 Solutions 4a (Chapter 4 problems) Chem151 [Kua]](http://s1.studyres.com/store/data/002731518_1-574ec10e88e667508364281b6325aeef-300x300.png)