... zone on the Greek island Syros in the Cycladic archipelago (Aegean Sea). The study is part of a PhD-project initiated by Prof. Dr. Alasdair Skelton and Dr. Iain Pitcairn (Stockholm University, Sweden) and Prof. Dr. Sandra Piazolo (Macquarie University, Australia). The PhD-project is focused on a bet ...
Stoichiometric Calculations
... 2 volumes of H2 plus 1 volume of O2 yields 2 volumes of H2 O. Note: The volume of a material is only proportional to the number of moles when the substance is in the gas phase! ...
... 2 volumes of H2 plus 1 volume of O2 yields 2 volumes of H2 O. Note: The volume of a material is only proportional to the number of moles when the substance is in the gas phase! ...
Changing Matter
... Making a mixture – 2 or more types of matter (substances) mixed together • Not in specific amounts • Can be separated physically ...
... Making a mixture – 2 or more types of matter (substances) mixed together • Not in specific amounts • Can be separated physically ...
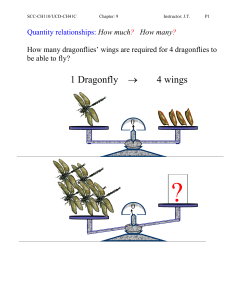
Quantity relationships: How much
... A mixture of 5.0 g of H2 (g) and 10.0 g of O2(g) is ignited. Water forms according to the following combination reaction: 2H2(g) +O2(g) → 2H2O(g) Which reactant is limiting? How much water will the reaction produce? ...
... A mixture of 5.0 g of H2 (g) and 10.0 g of O2(g) is ignited. Water forms according to the following combination reaction: 2H2(g) +O2(g) → 2H2O(g) Which reactant is limiting? How much water will the reaction produce? ...
AQA Science GCSE Chemistry
... AQA recognizes the importance of good-quality teaching, learning and assessment resources to accompany their specification. That's why they've chosen to work exclusively with nelson Thornes. With AQA examiners providing content and quality control, you can be confident that this course is as closely ...
... AQA recognizes the importance of good-quality teaching, learning and assessment resources to accompany their specification. That's why they've chosen to work exclusively with nelson Thornes. With AQA examiners providing content and quality control, you can be confident that this course is as closely ...
COMPETITION PTOBLEMS 1
... This publication contains the competition problems from the first twenty International Chemistry Olympiads (ICHO) organized in the years 1968 – 1988. It has been published by the ICHO International Information Centre in Bratislava (Slovakia) on the occasion of the 40th anniversary of this internatio ...
... This publication contains the competition problems from the first twenty International Chemistry Olympiads (ICHO) organized in the years 1968 – 1988. It has been published by the ICHO International Information Centre in Bratislava (Slovakia) on the occasion of the 40th anniversary of this internatio ...
Energetics
... depends only on the difference between the total enthalpy of the reactants and that of the products. ...
... depends only on the difference between the total enthalpy of the reactants and that of the products. ...
Soln Chem 2008Nov(9746)
... transitions cannot occur which accounts for both E and F being colourless. (ans) (ii) Cu3+ 1s2 2s2 2p6 3s2 3p6 3d8 Cu(III) in G has empty d-orbitals. Hence, d-d* electron transitions can take place and the light energy not absorbed is seen as the colour of the complex. (ans) © Step-by-Step ...
... transitions cannot occur which accounts for both E and F being colourless. (ans) (ii) Cu3+ 1s2 2s2 2p6 3s2 3p6 3d8 Cu(III) in G has empty d-orbitals. Hence, d-d* electron transitions can take place and the light energy not absorbed is seen as the colour of the complex. (ans) © Step-by-Step ...
AS/A Level Chemistry (A) specimen question papers and mark
... Specimen Question Papers and Mark Schemes These specimen assessment materials are designed to accompany the OCR Advanced Subsidiary GCE and Advanced GCE specifications in Chemistry for teaching from September 2000. Centres are permitted to copy material from this booklet for their own internal use. ...
... Specimen Question Papers and Mark Schemes These specimen assessment materials are designed to accompany the OCR Advanced Subsidiary GCE and Advanced GCE specifications in Chemistry for teaching from September 2000. Centres are permitted to copy material from this booklet for their own internal use. ...
Chemistry - Wheeling Jesuit University
... Chemistry is the central science linking mathematics and physics to the biological sciences.The creative insight of chemists into the substance of nature has led not only to an elegant model of the material world, but also to a valuable utility in everyday life. Our goal is to introduce students to ...
... Chemistry is the central science linking mathematics and physics to the biological sciences.The creative insight of chemists into the substance of nature has led not only to an elegant model of the material world, but also to a valuable utility in everyday life. Our goal is to introduce students to ...
Metallocene Organoactinide Complexes
... (η5 -C5 Me5 )3 M have unusually long metal ligand bonds. Although long metal ligand distances are known in f -element complexes containing agostic interactions [90], they generally involve only one or two interactions and the rest of the bonds are normal and predictable based on ionic radii [91–93]. ...
... (η5 -C5 Me5 )3 M have unusually long metal ligand bonds. Although long metal ligand distances are known in f -element complexes containing agostic interactions [90], they generally involve only one or two interactions and the rest of the bonds are normal and predictable based on ionic radii [91–93]. ...
Chapter 9 Review, pages 628–633
... of oxygen in its compounds is –2. Since there are 2 hydrogen atoms in H2C2O4(aq), the total contribution of the hydrogen atoms is +2. Since there are 4 oxygen atoms, the contribution of the oxygen atoms is –8. The 2 carbon atoms must have an oxidation number of +6 to give a sum of 0. Therefore, the ...
... of oxygen in its compounds is –2. Since there are 2 hydrogen atoms in H2C2O4(aq), the total contribution of the hydrogen atoms is +2. Since there are 4 oxygen atoms, the contribution of the oxygen atoms is –8. The 2 carbon atoms must have an oxidation number of +6 to give a sum of 0. Therefore, the ...
One-pot aqueous synthesis of cysteine-capped
... 2004; Kuno et al. 2006). These methods generally require the use of organic solvents, high temperature or pressure, oxygen and water-free conditions, and toxic reagents, such as three octyl phosphine (TOP three) and trioctylphosphine oxide (TOPO), all of which increase operational difficulty and pre ...
... 2004; Kuno et al. 2006). These methods generally require the use of organic solvents, high temperature or pressure, oxygen and water-free conditions, and toxic reagents, such as three octyl phosphine (TOP three) and trioctylphosphine oxide (TOPO), all of which increase operational difficulty and pre ...
44. Find рН of formic acid solution with mass percent ω=5
... 15. Write electronic structures of Be and B atoms in the ground and excited states. What are the valences of these elements? 16. Name elements – organogens. 17. Define biogenic role of calcium and magnesium. 18. Write electronic structures of Cu and Ag atoms. What families of elements do they belong ...
... 15. Write electronic structures of Be and B atoms in the ground and excited states. What are the valences of these elements? 16. Name elements – organogens. 17. Define biogenic role of calcium and magnesium. 18. Write electronic structures of Cu and Ag atoms. What families of elements do they belong ...