General chemistry laboratory activities, Lorentz
... accompanied by their necks, at the ends of which are ground glass joints to quickly and tightly connect to the rest of the apparatus (such as a reflux condenser or dropping funnel). The reaction flask is usually made of thick glass and they can tolerate large pressure differences, with the result th ...
... accompanied by their necks, at the ends of which are ground glass joints to quickly and tightly connect to the rest of the apparatus (such as a reflux condenser or dropping funnel). The reaction flask is usually made of thick glass and they can tolerate large pressure differences, with the result th ...
U6B _13-14
... Molecular Equation: the typical equation you are use to writing keeping all molecules together Complete Ionic Equation: shows all the particles in a solution as they really exist, as IONS or MOLECULES. Anything aqueous needs to be split apart into the cation and anion Anything solid stays intact ...
... Molecular Equation: the typical equation you are use to writing keeping all molecules together Complete Ionic Equation: shows all the particles in a solution as they really exist, as IONS or MOLECULES. Anything aqueous needs to be split apart into the cation and anion Anything solid stays intact ...
Topic 7.2 Equilibrium The Position of Equilibrium
... When you add something to a system at equilibrium, the system shifts in such a way as to use up what you’ve added. ...
... When you add something to a system at equilibrium, the system shifts in such a way as to use up what you’ve added. ...
Syllabus Cambridge IGCSE Chemistry (US) Syllabus Code 0439 For examination in 2013
... different IGCSE subject groups. It gives Centers the opportunity to benefit from offering a broad and balanced curriculum by recognizing the achievements of students who pass examinations in at least seven subjects, including two languages, and one subject from each of the other subject groups. The ...
... different IGCSE subject groups. It gives Centers the opportunity to benefit from offering a broad and balanced curriculum by recognizing the achievements of students who pass examinations in at least seven subjects, including two languages, and one subject from each of the other subject groups. The ...
Chapter 4 Classifying Reactions: Chemicals in Balance
... The names of the reactants and products are given. Plan Your Strategy In writing an equation the reactants are the starting materials and they are on the left side. The products are the new substances that form and they are on the right side. A plus sign, “+”, separate more than one reactant or prod ...
... The names of the reactants and products are given. Plan Your Strategy In writing an equation the reactants are the starting materials and they are on the left side. The products are the new substances that form and they are on the right side. A plus sign, “+”, separate more than one reactant or prod ...
File
... Chemistry 2202 - Unit 1 Test 2 Part 1: For each item, circle the letter corresponding to your choice. ...
... Chemistry 2202 - Unit 1 Test 2 Part 1: For each item, circle the letter corresponding to your choice. ...
APPROACHES TO CARBOHYDRATE-BASED CHEMICAL LIBRARIES: THE
... libraries must avoid redundancy and serve as a source of novel lead compounds with superior properties. Perhaps the stated purpose might be better phrased: "to produce superior new leads in drug discovery". This would entail the discovery of more lead structures, possessing higher affinities, signif ...
... libraries must avoid redundancy and serve as a source of novel lead compounds with superior properties. Perhaps the stated purpose might be better phrased: "to produce superior new leads in drug discovery". This would entail the discovery of more lead structures, possessing higher affinities, signif ...
Chapter 09 An Overview of Chemical Reactions Notes
... Precipitation Reaction: - a reaction where a precipitate (new solid) is formed as a product. Neutralization Reaction: - a reaction between an acid and a base where water is formed as a product. To Predict Products and Balance Chemical Equations: 1. Write the correct chemical formulas for all product ...
... Precipitation Reaction: - a reaction where a precipitate (new solid) is formed as a product. Neutralization Reaction: - a reaction between an acid and a base where water is formed as a product. To Predict Products and Balance Chemical Equations: 1. Write the correct chemical formulas for all product ...
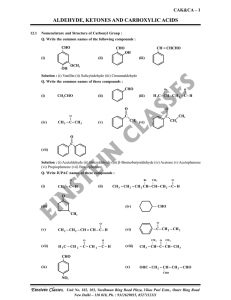
aldehyde, ketones and carboxylic acids
... Solution : A nucleophile attacks the electrophilic carbon atom of the polar carbonyl group from a direction approximately perpendicular to the plane of sp2 hybridised orbitals of carbonyl carbon. The hybridisation of carbon changes from sp2 to sp3 in this process, and a tetrahedral alkoxide intermed ...
... Solution : A nucleophile attacks the electrophilic carbon atom of the polar carbonyl group from a direction approximately perpendicular to the plane of sp2 hybridised orbitals of carbonyl carbon. The hybridisation of carbon changes from sp2 to sp3 in this process, and a tetrahedral alkoxide intermed ...
Supporting Information for Angew. Chem. Int. Ed. Z52444 © Wiley
... Division of Chemistry and Chemical Engineering California Institute of Technology 1200 E. California Blvd., MC 164-30, Pasadena CA 91125 (USA) FAX: (+1) 626-564-9297 E-mail: [email protected] Material and Methods. Unless stated otherwise, reactions were performed in oven-dried glassware, under an a ...
... Division of Chemistry and Chemical Engineering California Institute of Technology 1200 E. California Blvd., MC 164-30, Pasadena CA 91125 (USA) FAX: (+1) 626-564-9297 E-mail: [email protected] Material and Methods. Unless stated otherwise, reactions were performed in oven-dried glassware, under an a ...
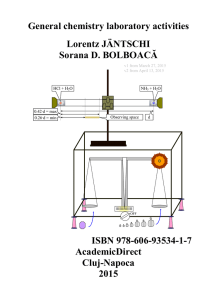
Laboratory Manual
... thoroughly in the sink. It can also be used if a person’s clothes catch on fire. Most safety showers are operated by pulling a chain. If a chemical is spilled on you so that you need to wash it off in a safety shower, let your teacher know and get there fast! The sooner the chemical is rinsed off, t ...
... thoroughly in the sink. It can also be used if a person’s clothes catch on fire. Most safety showers are operated by pulling a chain. If a chemical is spilled on you so that you need to wash it off in a safety shower, let your teacher know and get there fast! The sooner the chemical is rinsed off, t ...
study material(2014-15) class xii-chemistry
... Tips and Techniques for teaching/learning each chapter. Students‘ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among the ...
... Tips and Techniques for teaching/learning each chapter. Students‘ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among the ...
Modifying the stereochemistry of an enzyme
... here is a growing demand in the chemical and biotechnology industries for reliable and efficient methods for the production of enantiomerically pure compounds. Increasingly, synthetic chemists are exploiting enzymes in the asymmetric and stereoselective synthesis of chiral building blocks (1). Howev ...
... here is a growing demand in the chemical and biotechnology industries for reliable and efficient methods for the production of enantiomerically pure compounds. Increasingly, synthetic chemists are exploiting enzymes in the asymmetric and stereoselective synthesis of chiral building blocks (1). Howev ...
PPT - Gmu - George Mason University
... in the direction that increases the Entropy of the universe (universe = system + surroundings) Gibbs Free Energy (∆G) Difference between Enthalpy and the product of absolute temperature and the Entropy ...
... in the direction that increases the Entropy of the universe (universe = system + surroundings) Gibbs Free Energy (∆G) Difference between Enthalpy and the product of absolute temperature and the Entropy ...
chemistry-resource
... Tips and Techniques for teaching/learning each chapter. Students’ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among the ...
... Tips and Techniques for teaching/learning each chapter. Students’ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among the ...
Question Bank Topic 5
... (1) Used zinc-carbon cells can be disposed of in fire. (2) Zinc-carbon cells leak even though they are not in use. (3) The voltage of zinc-carbon cells drops rapidly over discharge. A (1) and (2) only B (1) and (3) only C (2) and (3) only D (1), (2) and (3) ...
... (1) Used zinc-carbon cells can be disposed of in fire. (2) Zinc-carbon cells leak even though they are not in use. (3) The voltage of zinc-carbon cells drops rapidly over discharge. A (1) and (2) only B (1) and (3) only C (2) and (3) only D (1), (2) and (3) ...
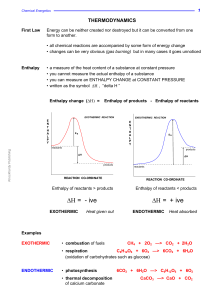
Η - Knockhardy
... the strength of a bond depends on its environment so MEAN values are quoted making a bond is an exothermic process as it is the opposite of breaking a bond for diatomic gases, the bond enthalpy is twice the enthalpy of atomisation the smaller the bond enthalpy, the weaker the bond and the easier it ...
... the strength of a bond depends on its environment so MEAN values are quoted making a bond is an exothermic process as it is the opposite of breaking a bond for diatomic gases, the bond enthalpy is twice the enthalpy of atomisation the smaller the bond enthalpy, the weaker the bond and the easier it ...
AS and A-level Chemistry Specification Specifications for first
... universities, to ensure these specifications allow students to develop the skills that they want to see. This approach has led to specifications that will help you to inspire students, nurture their passion for chemistry and lay the groundwork for further study in courses such as chemistry, medicine ...
... universities, to ensure these specifications allow students to develop the skills that they want to see. This approach has led to specifications that will help you to inspire students, nurture their passion for chemistry and lay the groundwork for further study in courses such as chemistry, medicine ...
g - mrnicholsscience
... • 25.0 g sucrose, C12H22O11 (FM=342g/mol), is burned, but only 30.0 g CO2 is recovered. • What is the percent yield? Do the mass-to-mass problem to find the expected yield. Divide the actual/expected, convert to a % ...
... • 25.0 g sucrose, C12H22O11 (FM=342g/mol), is burned, but only 30.0 g CO2 is recovered. • What is the percent yield? Do the mass-to-mass problem to find the expected yield. Divide the actual/expected, convert to a % ...
BSc (Hons) Chemistry (Optional Minor: Forensic Science)/MSc
... Chemistry is considered as the "central" science because it involves all of the other sciences. Chemistry seeks to understand the nature of matter in terms of atoms and molecules and the changes it undergoes. The mission of the Department of Chemistry is to provide students with the appropriate leve ...
... Chemistry is considered as the "central" science because it involves all of the other sciences. Chemistry seeks to understand the nature of matter in terms of atoms and molecules and the changes it undergoes. The mission of the Department of Chemistry is to provide students with the appropriate leve ...
1. Blood cholesterol levels are generally expressed as milligrams of
... ANSWER: Solution :Lets assume we have 1 mole of the each gas in the ballon at the STP conditions at STP condition 1 mole gas = 22.4 L so both balloons will have volume 22.4 L but the denities of the gases are different because mass of 1 mol N2 = 28.014 g per mol and molar mass of He = 4.0026 g per m ...
... ANSWER: Solution :Lets assume we have 1 mole of the each gas in the ballon at the STP conditions at STP condition 1 mole gas = 22.4 L so both balloons will have volume 22.4 L but the denities of the gases are different because mass of 1 mol N2 = 28.014 g per mol and molar mass of He = 4.0026 g per m ...
Review Study Guide for the Final
... To what volume, in liters, must you dilute a solution containing 4 liters of 0.100 M of Ca(OH)2 to obtain a 0.00100 M solution as calcium hydroxide (Ca(OH)2)? ...
... To what volume, in liters, must you dilute a solution containing 4 liters of 0.100 M of Ca(OH)2 to obtain a 0.00100 M solution as calcium hydroxide (Ca(OH)2)? ...