Thermochemistry Diploma Questions
... a. An endothermic chemical reaction, which absorbs energy and increases the potential energy of the water, occurred. b. An endothermic chemical reaction, which absorbs energy and increases the kinetic energy of the water, occurred. c. An exothermic chemical reaction, which releases energy and increa ...
... a. An endothermic chemical reaction, which absorbs energy and increases the potential energy of the water, occurred. b. An endothermic chemical reaction, which absorbs energy and increases the kinetic energy of the water, occurred. c. An exothermic chemical reaction, which releases energy and increa ...
Chemical Reactions - 2012 Book Archive
... antibiotics such as amoxicillin, were unknown only a few years ago. Their development required that chemists understand how substances combine in certain ratios and under specific conditions to produce a new substance with particular properties. ...
... antibiotics such as amoxicillin, were unknown only a few years ago. Their development required that chemists understand how substances combine in certain ratios and under specific conditions to produce a new substance with particular properties. ...
Article PDF - IOPscience
... metallicity dependence of XPS enters only through the PH3 and H2S abundances, whereas the H2 abundance is metallicity independent by definition. Phosphine and H2S are typically the most abundant P- and S-bearing gases, respectively. Hence, XPH3 and XH2 S must each be expanded with a metallicity fact ...
... metallicity dependence of XPS enters only through the PH3 and H2S abundances, whereas the H2 abundance is metallicity independent by definition. Phosphine and H2S are typically the most abundant P- and S-bearing gases, respectively. Hence, XPH3 and XH2 S must each be expanded with a metallicity fact ...
LABORATORY MANUAL FOR CHEMISTRY 102
... where [A], [B], [C], ... represent molarities of all chemical species that affect the rate, and x, y, z, ... are the experimentally determined exponents for each species. (The overall order of the reaction is equal to the sum of x + y + z +... .) The term k is known as the rate constant for the reac ...
... where [A], [B], [C], ... represent molarities of all chemical species that affect the rate, and x, y, z, ... are the experimentally determined exponents for each species. (The overall order of the reaction is equal to the sum of x + y + z +... .) The term k is known as the rate constant for the reac ...
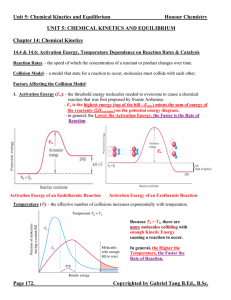
Unit 5: Chemical Kinetics and Equilibrium
... at the state of equilibrium. ([C]eq and [D]eq or PC, eq and PD, eq >> [A]eq and [B]eq or PA, eq and PB, eq) 2. When K << 1, the equilibrium system favours the reactants. There are less products than reactants at the state of equilibrium. ([A]eq and [B]eq or PA, eq and PB, eq >> [C]eq and [D]eq or PC ...
... at the state of equilibrium. ([C]eq and [D]eq or PC, eq and PD, eq >> [A]eq and [B]eq or PA, eq and PB, eq) 2. When K << 1, the equilibrium system favours the reactants. There are less products than reactants at the state of equilibrium. ([A]eq and [B]eq or PA, eq and PB, eq >> [C]eq and [D]eq or PC ...
Introduction to Inorganic Chemistry
... (ii) the utilization of material change for human ends. Ideally, the first activity provides the necessary know-how for the pursuit of the second, but in practice, the help it can give is only partial, and the second activity has to fall back on trial and error techniques in order to achieve its end ...
... (ii) the utilization of material change for human ends. Ideally, the first activity provides the necessary know-how for the pursuit of the second, but in practice, the help it can give is only partial, and the second activity has to fall back on trial and error techniques in order to achieve its end ...
indian association of chemistry teachers
... Semipermeable nature of the cell membrane can be attributed to the presence of CAREER POINT, CP Tower, Road No.1, IPIA, Kota (Raj.) Ph.: 0744-3040000 Website : www.careerpointgroup.com, Email: [email protected] ...
... Semipermeable nature of the cell membrane can be attributed to the presence of CAREER POINT, CP Tower, Road No.1, IPIA, Kota (Raj.) Ph.: 0744-3040000 Website : www.careerpointgroup.com, Email: [email protected] ...
biomolecules (introduction, structure
... Here, we observe that the amino group on the asymmetric carbon atom of alanine can be stereochemically related to the substituent hydroxyl group on the asymmetric carbon atom of glyceraldehyde, the carboxyl group of alanine can be related to the aldehyde group of glyceraldehyde, and the R group of a ...
... Here, we observe that the amino group on the asymmetric carbon atom of alanine can be stereochemically related to the substituent hydroxyl group on the asymmetric carbon atom of glyceraldehyde, the carboxyl group of alanine can be related to the aldehyde group of glyceraldehyde, and the R group of a ...
Rh(acac)(CO)(PR1R2R3) - University of the Free State
... octahedral geometries. The oxidative addition and reductive elimination reactions from Rh(I) to Rh(III) and vice versa, has transformed the catalytic industry and has produced many fascinating reactions over the years. ...
... octahedral geometries. The oxidative addition and reductive elimination reactions from Rh(I) to Rh(III) and vice versa, has transformed the catalytic industry and has produced many fascinating reactions over the years. ...
STUDY MATERIAL 2016-17 CHEMISTRY CLASS XII
... 4. Classify each of the following as either a p-type or n-type semi-conductor. a) Ge doped with In b) Si doped with P (a) Ge is group 14 element and In is group 13 element. Therefore, an electron deficient hole is created. Thus semi-conductor is p-type. (b) Since P is a group 15 element and Si is gr ...
... 4. Classify each of the following as either a p-type or n-type semi-conductor. a) Ge doped with In b) Si doped with P (a) Ge is group 14 element and In is group 13 element. Therefore, an electron deficient hole is created. Thus semi-conductor is p-type. (b) Since P is a group 15 element and Si is gr ...
Stoichiometry PP
... The heat of a reaction can be calculated by subtracting the heats of formation of the reactants from the products ...
... The heat of a reaction can be calculated by subtracting the heats of formation of the reactants from the products ...
Massachusetts Tests for Educator Licensure (MTEL )
... Correct Response: C. Nuclear fission results in radioactive waste products that need to be stored long-term. Nuclear power plants are extremely expensive to build and represent a variety of safety hazards. However, greenhouse gases such as carbon dioxide are not produced as a result of using nuclear ...
... Correct Response: C. Nuclear fission results in radioactive waste products that need to be stored long-term. Nuclear power plants are extremely expensive to build and represent a variety of safety hazards. However, greenhouse gases such as carbon dioxide are not produced as a result of using nuclear ...
equilibrium - eVirtualGuru
... H2O (l) ⇌ H2O (vap) The double half arrows indicate that the processes in both the directions are going on simultaneously. The mixture of reactants and products in the equilibrium state is called an equilibrium mixture. Equilibrium can be established for both physical processes and chemical reaction ...
... H2O (l) ⇌ H2O (vap) The double half arrows indicate that the processes in both the directions are going on simultaneously. The mixture of reactants and products in the equilibrium state is called an equilibrium mixture. Equilibrium can be established for both physical processes and chemical reaction ...
Problem 1-2 - IPN-Kiel
... Draw the structural formulae of A, B and C and show how you derived the structures from the hints and the reaction equation. Which kind of isomers are formed in the reaction 1 A? Draw the structural formulae! Analyze the 1H-NMR spectrum of A: Assign all hydrogen atoms of b) to the corresponding si ...
... Draw the structural formulae of A, B and C and show how you derived the structures from the hints and the reaction equation. Which kind of isomers are formed in the reaction 1 A? Draw the structural formulae! Analyze the 1H-NMR spectrum of A: Assign all hydrogen atoms of b) to the corresponding si ...
Spontaneous Change: Entropy and Gibbs Energy
... The modern interpretation of entropy is firmly rooted in the idea that a macroscopic system is made up of many particles (often 1023 or more). Consider, for example, a fixed amount, n, of an ideal gas at temperature T in a container of volume V. The pressure of the gas is P = nRT/V. At the macroscop ...
... The modern interpretation of entropy is firmly rooted in the idea that a macroscopic system is made up of many particles (often 1023 or more). Consider, for example, a fixed amount, n, of an ideal gas at temperature T in a container of volume V. The pressure of the gas is P = nRT/V. At the macroscop ...
Introduction to Inorganic Chemistry
... (ii) the utilization of material change for human ends. Ideally, the first activity provides the necessary know-how for the pursuit of the second, but in practice, the help it can give is only partial, and the second activity has to fall back on trial and error techniques in order to achieve its end ...
... (ii) the utilization of material change for human ends. Ideally, the first activity provides the necessary know-how for the pursuit of the second, but in practice, the help it can give is only partial, and the second activity has to fall back on trial and error techniques in order to achieve its end ...
Chemical Dynamics at Surfaces
... One of the most important discoveries in the field of chemistry in the last 100 years N2+ 3H2→2NH3 ...
... One of the most important discoveries in the field of chemistry in the last 100 years N2+ 3H2→2NH3 ...
Quarter 1
... 5. Ernest Rutherford performed an experiment in which he shot alpha particles through a thin layer of gold foil. He predicted that the alpha particles would travel straight through the gold ...
... 5. Ernest Rutherford performed an experiment in which he shot alpha particles through a thin layer of gold foil. He predicted that the alpha particles would travel straight through the gold ...