
advanced placement chemistry workbook and note set
... The name of a particular isotope is the element name plus its mass number. The name of the isotope on the far left in Figure 4 is carbon-12, the middle isotope is carbon-13 and the isotope on the right is carbon-14. This system of naming isotopes helps distinguish between the varying Figure 4. You c ...
... The name of a particular isotope is the element name plus its mass number. The name of the isotope on the far left in Figure 4 is carbon-12, the middle isotope is carbon-13 and the isotope on the right is carbon-14. This system of naming isotopes helps distinguish between the varying Figure 4. You c ...
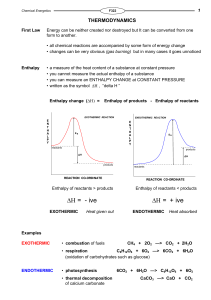
Enthalpy change
... Standard Enthalpy Changes • enthalpy values vary with the conditions - so standard conditions are needed • a substance will then be in its standard state ... Pressure:- 100 kPa (1 atm) ...
... Standard Enthalpy Changes • enthalpy values vary with the conditions - so standard conditions are needed • a substance will then be in its standard state ... Pressure:- 100 kPa (1 atm) ...
Teaching with CAChe - Photochemical Dynamics Group
... CAChe has become a normal tool which we rarely think of as an unusual feature of our program. The addition of molecular modeling into our curriculum was more feasible than many other settings. Our classes were relatively small, and we had flexibility in scheduling non-class time for individual stude ...
... CAChe has become a normal tool which we rarely think of as an unusual feature of our program. The addition of molecular modeling into our curriculum was more feasible than many other settings. Our classes were relatively small, and we had flexibility in scheduling non-class time for individual stude ...
Document
... chemical that makes the least amount of product is the “limiting reagent”. You can recognize limiting reagent problems because they will give you 2 amounts of chemical Do two stoichiometry problems, one for each reagent you are given. ...
... chemical that makes the least amount of product is the “limiting reagent”. You can recognize limiting reagent problems because they will give you 2 amounts of chemical Do two stoichiometry problems, one for each reagent you are given. ...
Chapter 8 - Chemical Equations and Reactions
... Section 2 Types of Chemical Reactions Section 3 Activity Series of the Elements ...
... Section 2 Types of Chemical Reactions Section 3 Activity Series of the Elements ...
THESE DOCTORAT DE L`UNIVERSITE DE TOULOUSE ET
... shows a greater tendency of the Mo compound to be reduced. For the W complex, on the other hand, a reversible reaction gives rise to a simple Cp*WVI ligand exchange product. The corresponding reaction for thioglycolic acid resulted in an adduct having the same stoichiometry at low substrate/W ratio, ...
... shows a greater tendency of the Mo compound to be reduced. For the W complex, on the other hand, a reversible reaction gives rise to a simple Cp*WVI ligand exchange product. The corresponding reaction for thioglycolic acid resulted in an adduct having the same stoichiometry at low substrate/W ratio, ...
Stoichiometry
... formed. The reaction will stop when all of the limiting reactant is consumed. Example: I want to assemble a gadget that requires one nut, one bolt and two washers for every hole. I have in my garage a bucket filled with 12 washers, 4 bolts and five nuts. What is the LIMITING SMALL METAL ...
... formed. The reaction will stop when all of the limiting reactant is consumed. Example: I want to assemble a gadget that requires one nut, one bolt and two washers for every hole. I have in my garage a bucket filled with 12 washers, 4 bolts and five nuts. What is the LIMITING SMALL METAL ...
Module 1 Predictor Questions
... It is important to recognize that these prefixes may be used with any unit of measurement, and that the relationship between the base unit and the unit with the prefix is always the same regardless of the base unit. The base unit is represented by x in the table. Pay special attention to the unit fa ...
... It is important to recognize that these prefixes may be used with any unit of measurement, and that the relationship between the base unit and the unit with the prefix is always the same regardless of the base unit. The base unit is represented by x in the table. Pay special attention to the unit fa ...
Elements Of Physical Chemistry 4th Edition Laidler
... physical chemistry 4th edition amazon com - buy physical chemistry on amazon com the si iupac recommendations appendix b physical constants appendix c some this is about 4th edition by laidler, 0618123423 physical chemistry solutions manual 4th - physical chemistry solutions manual 4th edition keith ...
... physical chemistry 4th edition amazon com - buy physical chemistry on amazon com the si iupac recommendations appendix b physical constants appendix c some this is about 4th edition by laidler, 0618123423 physical chemistry solutions manual 4th - physical chemistry solutions manual 4th edition keith ...
Mock Examination (2016/2017) CHEMISTRY PAPER 1 SECTION B
... The Cu2+ in the electrolyte is a stronger oxidizing agent than H + in electrochemical series. (1M) The Cu2+ will be preferentially discharged and is reduced to Cu, and Cu in cathode electrode will not undergo oxidation to form Cu2+ thus the concentration of Cu2+ is decreased. (1M) ...
... The Cu2+ in the electrolyte is a stronger oxidizing agent than H + in electrochemical series. (1M) The Cu2+ will be preferentially discharged and is reduced to Cu, and Cu in cathode electrode will not undergo oxidation to form Cu2+ thus the concentration of Cu2+ is decreased. (1M) ...
Document
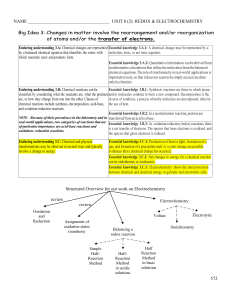
... Learning objective 3.1 Students can translate among macroscopic observations of change, chemical equations, and particle views. [See SP 1.5, 7.1; Essential knowledge components of 3.A–3.C] Learning objective 3.2 The student can translate an observed chemical change into a balanced chemical equation ...
... Learning objective 3.1 Students can translate among macroscopic observations of change, chemical equations, and particle views. [See SP 1.5, 7.1; Essential knowledge components of 3.A–3.C] Learning objective 3.2 The student can translate an observed chemical change into a balanced chemical equation ...
EIT Review S2012 Part 2 Dr. J. Mack CSUS Department of Chemistry
... • Concentration data can be used to calculate equilibrium constants for both aqueous and gaseous systems. • In these cases, the symbol K is sometimes given the subscript “c” for “concentration,” as in Kc. • For gases, however, equilibrium constant expressions can be written in another way: in ...
... • Concentration data can be used to calculate equilibrium constants for both aqueous and gaseous systems. • In these cases, the symbol K is sometimes given the subscript “c” for “concentration,” as in Kc. • For gases, however, equilibrium constant expressions can be written in another way: in ...
Chemistry 134 Problem Set Introduction
... 14.38 (a) What is the difference between a sapphire and a ruby? (b) Why might aluminum be present with silicon in many minerals? 14.39 (a) List the stable oxidation states for each member of the boron family. (b) For any element that may have more than one stable oxidation state, identify the more s ...
... 14.38 (a) What is the difference between a sapphire and a ruby? (b) Why might aluminum be present with silicon in many minerals? 14.39 (a) List the stable oxidation states for each member of the boron family. (b) For any element that may have more than one stable oxidation state, identify the more s ...
Slide 1
... Write the balanced equation for the reaction that occurs when methanol, CH 3OH(l), is burned in air. Solution When any compound containing C, H, and O is combusted, it reacts with the O 2(g) in air to produce CO2(g) and H2O(g). Thus, the unbalanced equation is CH3OH(l) + O2(g) → CO2(g) + H2O(g) In t ...
... Write the balanced equation for the reaction that occurs when methanol, CH 3OH(l), is burned in air. Solution When any compound containing C, H, and O is combusted, it reacts with the O 2(g) in air to produce CO2(g) and H2O(g). Thus, the unbalanced equation is CH3OH(l) + O2(g) → CO2(g) + H2O(g) In t ...
Review Unit: Chemistry Review
... possible. Science would not progress very far without the increasingly advanced technologies available to scientists. Often scientific advances have to wait on the development of technologies for research to be done; for example, glassware, the battery, the laser, and the computer. Often science is ...
... possible. Science would not progress very far without the increasingly advanced technologies available to scientists. Often scientific advances have to wait on the development of technologies for research to be done; for example, glassware, the battery, the laser, and the computer. Often science is ...
Document
... do not appear in the equilibrium constant expressions. 3. The equilibrium constant is a dimensionless quantity. 4. In quoting a value for the equilibrium constant, you must specify the balanced equation and the temperature. 5. If a reaction can be expressed as a sum of two or more reactions, the equ ...
... do not appear in the equilibrium constant expressions. 3. The equilibrium constant is a dimensionless quantity. 4. In quoting a value for the equilibrium constant, you must specify the balanced equation and the temperature. 5. If a reaction can be expressed as a sum of two or more reactions, the equ ...
Problem Authors - PianetaChimica
... for the 43rd International Chemistry Olympiad easier for both students and mentors. We restricted ourselves to the inclusion of only a few topics that are not usually covered in secondary schools. There are six such advanced topics in theoretical part that we expect the participants to be familiar w ...
... for the 43rd International Chemistry Olympiad easier for both students and mentors. We restricted ourselves to the inclusion of only a few topics that are not usually covered in secondary schools. There are six such advanced topics in theoretical part that we expect the participants to be familiar w ...
chap15pptlecture_chapte.ppt [Read-Only]
... If a reaction can be expressed as the sum of two or more reactions, the equilibrium constant for the overall reaction is given by the product of the equilibrium constants of the individual reactions. ...
... If a reaction can be expressed as the sum of two or more reactions, the equilibrium constant for the overall reaction is given by the product of the equilibrium constants of the individual reactions. ...
AS/A level
... 1 mol of a gas occupies 24.0 dm3 at 298 K and 1 atm. Calculate the temperature at which it occupies 25.0 dm3 at the same pressure. ...
... 1 mol of a gas occupies 24.0 dm3 at 298 K and 1 atm. Calculate the temperature at which it occupies 25.0 dm3 at the same pressure. ...




















![chap15pptlecture_chapte.ppt [Read-Only]](http://s1.studyres.com/store/data/015369082_1-00cbf06a2d468a4ae1c963f5ca674e31-300x300.png)
