Chemistry
... of oxymercuration-demercuration, hydroboration- oxidation, ozonolysis, reduction (catalytic and chemical), syn and anti-hydroxylation(oxidation). 1,2-and 1,4-addition reactions in conjugated dienes and Diels-Alder reaction; Allylic and benzylic bromination and mechanism, e.g. propene, 1-butene, tolu ...
... of oxymercuration-demercuration, hydroboration- oxidation, ozonolysis, reduction (catalytic and chemical), syn and anti-hydroxylation(oxidation). 1,2-and 1,4-addition reactions in conjugated dienes and Diels-Alder reaction; Allylic and benzylic bromination and mechanism, e.g. propene, 1-butene, tolu ...
UNIVERSITY OF DELHI FACULTY OF SCIENCE SYLLABUS OF COURSES TO BE OFFERED
... provides an extended scope or which enables an exposure to some other discipline/subject/domain or nurtures the candidate’s proficiency/skill is called an Elective Course. 2.1 Discipline Specific Elective (DSE) Course: Elective courses may be offered by the main discipline/subject of study is referr ...
... provides an extended scope or which enables an exposure to some other discipline/subject/domain or nurtures the candidate’s proficiency/skill is called an Elective Course. 2.1 Discipline Specific Elective (DSE) Course: Elective courses may be offered by the main discipline/subject of study is referr ...
Thermodynamics: Entropy and Free Energy
... The third law of thermodynamics allows us to determine the entropy of a substance at a given temperature, or its standard molar entropy (Sº). The standard molar entropy of a pure substance can be determined by careful measurements of its molar heat capacity as a function of temperature and of the he ...
... The third law of thermodynamics allows us to determine the entropy of a substance at a given temperature, or its standard molar entropy (Sº). The standard molar entropy of a pure substance can be determined by careful measurements of its molar heat capacity as a function of temperature and of the he ...
1aUnit Two Handouts - Dunmore High School
... What is the role of water in this reaction? Explain ...
... What is the role of water in this reaction? Explain ...
Organic Chemistry Organic Chemistry
... hydrogen bonds not only further increases intermolecular attractions, it also enables these molecules to mix readily with polar solutes and solvents. You may recall the saying “like dissolves like.” The solubility of organic compounds is affected by nonpolar components and polar components within th ...
... hydrogen bonds not only further increases intermolecular attractions, it also enables these molecules to mix readily with polar solutes and solvents. You may recall the saying “like dissolves like.” The solubility of organic compounds is affected by nonpolar components and polar components within th ...
The Role of Medicinal Chemistry in Canadian Pharmacy
... “Helps to remember drugs for THERAPEUTICS” CPERC-2013, Praveen P Nekkar Rao, UW Pharmacy ...
... “Helps to remember drugs for THERAPEUTICS” CPERC-2013, Praveen P Nekkar Rao, UW Pharmacy ...
File - UTeach Dallas Project
... emphasis on factual material and greater emphasis on understanding and application of scientific concepts and principles. This has been done so that learners develop skills that will be of the value for a long time in an increasingly world and it is expected that these will be of relevance for a ver ...
... emphasis on factual material and greater emphasis on understanding and application of scientific concepts and principles. This has been done so that learners develop skills that will be of the value for a long time in an increasingly world and it is expected that these will be of relevance for a ver ...
Exam Edge Digital
... The Chemical Bonding: Chemical Formulas chapter is important in that it teaches you several fundamental principles that apply to many aspects of chemistry. Questions on this chapter appear frequently in questions 4, 5, 10 and 11 of the exam paper. You should be able to draw diagrams to show the elec ...
... The Chemical Bonding: Chemical Formulas chapter is important in that it teaches you several fundamental principles that apply to many aspects of chemistry. Questions on this chapter appear frequently in questions 4, 5, 10 and 11 of the exam paper. You should be able to draw diagrams to show the elec ...
chemistry - The Aga Khan University
... 10.1.2 Hydrophilic and Hydrophobic Molecules 10.1.3 The Nature of Solutions in Liquid Phase 10.1.4 The Effect of Temperature and Pressure on Solubility ...
... 10.1.2 Hydrophilic and Hydrophobic Molecules 10.1.3 The Nature of Solutions in Liquid Phase 10.1.4 The Effect of Temperature and Pressure on Solubility ...
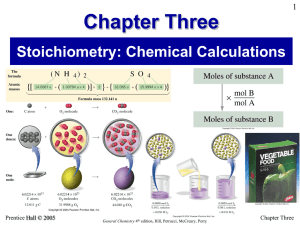
Stoichiometry: Calculations with Chemical Formulas and Equations
... Sample Exercise 3.19 Calculating the Amount of Product Formed from a Limiting Reactant Consider the following reaction that occurs in a fuel cell: 2 H2(g) + O2 (g) → 2 H2O (g) This reaction, properly done, produces energy in the form of electricity and water. Suppose a fuel cell is set up with 150 ...
... Sample Exercise 3.19 Calculating the Amount of Product Formed from a Limiting Reactant Consider the following reaction that occurs in a fuel cell: 2 H2(g) + O2 (g) → 2 H2O (g) This reaction, properly done, produces energy in the form of electricity and water. Suppose a fuel cell is set up with 150 ...
THE STUDY OF INTERMEDIARY METABOLISM OF
... has been derived from A. This conclusion, however, is valid only if the deuterium content in the stable hydrogen of B is higher t,han that, of the body fluids. The administration of deuterium compounds generally leads to the formation, due to their biological degradation, of some heavy water, which ...
... has been derived from A. This conclusion, however, is valid only if the deuterium content in the stable hydrogen of B is higher t,han that, of the body fluids. The administration of deuterium compounds generally leads to the formation, due to their biological degradation, of some heavy water, which ...
redox reaction - Seattle Central College
... Earlier in the quarter we defined a solution as a homogeneous mixture; a random combination of two or more things. The part of the solution we have the most of is the solvent and the minor components of a solution are referred to as the solutes. Water is the most common solvent and a good one for io ...
... Earlier in the quarter we defined a solution as a homogeneous mixture; a random combination of two or more things. The part of the solution we have the most of is the solvent and the minor components of a solution are referred to as the solutes. Water is the most common solvent and a good one for io ...
2014 International Practice Exam: Chemistry
... Students .are .not .allowed .to .use .calculators .in .Section .I .of .the .AP .Chemistry .Exam . .However, . students .are .permitted .to .use .four-function, .scientific, .or .graphing .calculators .to .answer . questions .in .Section .II . .Before .starting .the .exam .administration, .make .sure ...
... Students .are .not .allowed .to .use .calculators .in .Section .I .of .the .AP .Chemistry .Exam . .However, . students .are .permitted .to .use .four-function, .scientific, .or .graphing .calculators .to .answer . questions .in .Section .II . .Before .starting .the .exam .administration, .make .sure ...
Packet 1 - Kentucky Community and Technical College System
... Well, ¾ of the Earth is covered by water. And, 70-80% of us is water. Because water is so abundant it is very useful to use as a solvent. The fact that so many ionic and molecular chemicals are soluble in it makes it even more useful. ...
... Well, ¾ of the Earth is covered by water. And, 70-80% of us is water. Because water is so abundant it is very useful to use as a solvent. The fact that so many ionic and molecular chemicals are soluble in it makes it even more useful. ...
Removal of Chlorine Removal of Chlorine
... chlorine gases from streams of air. TEDA is necessary to promote hydrolysis reactions involving Cl2 and COCl2 Terminal hydroxyl groups associated with Zr(OH)4 contribute to the removal of HCl and product HCl (from hydrolysis of Cl2 or COCl2) Addition of zinc to the formulation greatly improves the r ...
... chlorine gases from streams of air. TEDA is necessary to promote hydrolysis reactions involving Cl2 and COCl2 Terminal hydroxyl groups associated with Zr(OH)4 contribute to the removal of HCl and product HCl (from hydrolysis of Cl2 or COCl2) Addition of zinc to the formulation greatly improves the r ...
2016-2018 Syllabus - Cambridge International Examinations
... final result.’ Page 39: Section C5.3.4 Suggesting improvements, paragraph after list, 6th line, now reads ‘may relate to sources of error or uncertainty identified by the candidate or to other sources of error or uncertainty.’ The changes below were introduced for version 1 of this syllabus. Part of ...
... final result.’ Page 39: Section C5.3.4 Suggesting improvements, paragraph after list, 6th line, now reads ‘may relate to sources of error or uncertainty identified by the candidate or to other sources of error or uncertainty.’ The changes below were introduced for version 1 of this syllabus. Part of ...
Manual Physical Chemistry III
... Experimental 1: Determination of Surface Tension of Liquids by drop weight Method ............................................ 2 Experimental 2: Determination of Surface Tension Liquids by Capillary Rise Method ............................................... 8 Experimental 3: To find out the partiti ...
... Experimental 1: Determination of Surface Tension of Liquids by drop weight Method ............................................ 2 Experimental 2: Determination of Surface Tension Liquids by Capillary Rise Method ............................................... 8 Experimental 3: To find out the partiti ...
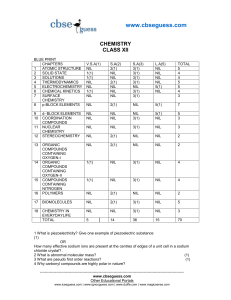
guess paper class xii
... (i) 238U92 undergoes decay (ii) 234Pa91 undergoes - decay (iii) 22Na11 undergoes + decay ...
... (i) 238U92 undergoes decay (ii) 234Pa91 undergoes - decay (iii) 22Na11 undergoes + decay ...
Now! - Soojeede.com
... A great number of people associate a strong acid with its ability to react with skin, essentially “melting´ it away from bone. It was only recently on a popular crime show that this very acid chemistry know-how was ...
... A great number of people associate a strong acid with its ability to react with skin, essentially “melting´ it away from bone. It was only recently on a popular crime show that this very acid chemistry know-how was ...