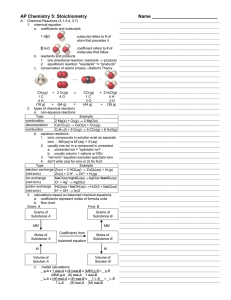
Chapter 1 – Reaction Kinetics Answer Key
... 3. The concentrations of pure solids and liquids are fixed. That is they do not change (appreciably for the liquid if it is the solvent and at all for the solid) during a chemical reaction. ...
... 3. The concentrations of pure solids and liquids are fixed. That is they do not change (appreciably for the liquid if it is the solvent and at all for the solid) during a chemical reaction. ...
B.Sc. (Hons.) Chemistry
... 6. Organometallics, Bioinorganic chemistry, Polynuclear hydrocarbons and UV, IR Spectroscopy 7. Molecules of life (4) + Lab (4). Note: Universities may include more options or delete some from this list Important: 1. Each University/Institute should provide a brief write-up about each paper outlinin ...
... 6. Organometallics, Bioinorganic chemistry, Polynuclear hydrocarbons and UV, IR Spectroscopy 7. Molecules of life (4) + Lab (4). Note: Universities may include more options or delete some from this list Important: 1. Each University/Institute should provide a brief write-up about each paper outlinin ...
Chemistry
... This course deals with the major topics of concern in environmental chemistry. Emphasis is placed on the chemistry involved, as well as assessment of the relative hazards and corrective methods available to provide abatement. Topics covered include: atmospheric free radical chemistry, the green- hou ...
... This course deals with the major topics of concern in environmental chemistry. Emphasis is placed on the chemistry involved, as well as assessment of the relative hazards and corrective methods available to provide abatement. Topics covered include: atmospheric free radical chemistry, the green- hou ...
AP Chemistry
... grams of a product from the grams of a reactant, first convert grams of reactant to moles of reactant, then use the coefficients to convert the number of moles of reactant to moles of product, and finally convert moles of product to grams of product. Many reactions occur in water (aqueous). Molarity ...
... grams of a product from the grams of a reactant, first convert grams of reactant to moles of reactant, then use the coefficients to convert the number of moles of reactant to moles of product, and finally convert moles of product to grams of product. Many reactions occur in water (aqueous). Molarity ...
Stoichiometry - coercingmolecules
... b. Consider a 500.-mg tablet. How many moles of sodium ascorbate are present? c. How many moles of C are present? d. How many moles of Na are present? e. How many formula units of sodium ascorbate are present? f. How many atoms of Na are present? ...
... b. Consider a 500.-mg tablet. How many moles of sodium ascorbate are present? c. How many moles of C are present? d. How many moles of Na are present? e. How many formula units of sodium ascorbate are present? f. How many atoms of Na are present? ...
STUDY GUIDE
... MAIN IDEA: Hydrocarbons with multiple bonds are more reactive than alkanes and participate in addition reactions in which atoms from one molecule are added to another molecule. Addition reactions include hydrogenation, halogenation, hydrohalogenation, and hydration. Markovnikov’s rule states that, ...
... MAIN IDEA: Hydrocarbons with multiple bonds are more reactive than alkanes and participate in addition reactions in which atoms from one molecule are added to another molecule. Addition reactions include hydrogenation, halogenation, hydrohalogenation, and hydration. Markovnikov’s rule states that, ...
Equilibrium - AP Chemistry
... The Concept of Equilibrium • Consider colorless frozen N2O4. At room temperature, it decomposes to brown NO2: N2O4(g) 2NO2(g). • At some time, the color stops changing and we have a mixture of N2O4 and NO2. • Chemical equilibrium is the point at which the rate of the forward reaction is equal to ...
... The Concept of Equilibrium • Consider colorless frozen N2O4. At room temperature, it decomposes to brown NO2: N2O4(g) 2NO2(g). • At some time, the color stops changing and we have a mixture of N2O4 and NO2. • Chemical equilibrium is the point at which the rate of the forward reaction is equal to ...
CBSE Living Science Chemistry Class X
... Dr Syamal has published about 350 scientific research papers, some of which are in the field of improvement of science education at the high school level. He has received awards from American Chemical Society for his contribution towards the development of science and technology in the last four dec ...
... Dr Syamal has published about 350 scientific research papers, some of which are in the field of improvement of science education at the high school level. He has received awards from American Chemical Society for his contribution towards the development of science and technology in the last four dec ...
Equilibrium - Tenafly High School
... The Concept of Equilibrium • Consider colorless frozen N2O4. At room temperature, it decomposes to brown NO2: N2O4(g) 2NO2(g). • At some time, the color stops changing and we have a mixture of N2O4 and NO2. • Chemical equilibrium is the point at which the rate of the forward reaction is equal to ...
... The Concept of Equilibrium • Consider colorless frozen N2O4. At room temperature, it decomposes to brown NO2: N2O4(g) 2NO2(g). • At some time, the color stops changing and we have a mixture of N2O4 and NO2. • Chemical equilibrium is the point at which the rate of the forward reaction is equal to ...
The Equilibrium Constant
... The Concept of Equilibrium • Consider colorless frozen N2O4. At room temperature, it decomposes to brown NO2: N2O4(g) 2NO2(g). • At some time, the color stops changing and we have a mixture of N2O4 and NO2. • Chemical equilibrium is the point at which the rate of the forward reaction is equal to ...
... The Concept of Equilibrium • Consider colorless frozen N2O4. At room temperature, it decomposes to brown NO2: N2O4(g) 2NO2(g). • At some time, the color stops changing and we have a mixture of N2O4 and NO2. • Chemical equilibrium is the point at which the rate of the forward reaction is equal to ...
Last Name Professor BEAMER First Name
... You are converting between particles (molecules) and mass (grams). Therefore, you need to use Avogadro’s Number. ...
... You are converting between particles (molecules) and mass (grams). Therefore, you need to use Avogadro’s Number. ...
OCR AS Level Chemistry B (Salters) H033
... Chemistry B (Salters) was first examined in 1992 as a new concept project examination. In contrast to the traditional ‘topic-based’ approach, Chemistry B (Salters) is ‘context-led’. Chemical concepts are introduced within a relevant context, the course being written as a series of teaching modules b ...
... Chemistry B (Salters) was first examined in 1992 as a new concept project examination. In contrast to the traditional ‘topic-based’ approach, Chemistry B (Salters) is ‘context-led’. Chemical concepts are introduced within a relevant context, the course being written as a series of teaching modules b ...
DEPARTMENT OF CHEMISTRY Requirements For Chemistry Major
... Courses For Chemistry Major All core Chemistry Courses for all the years must be taken by students intending to major in Chemistry. In addition, a useful selection of Elective Courses should be made in order to augment the depth of the subject expected of a Major. ...
... Courses For Chemistry Major All core Chemistry Courses for all the years must be taken by students intending to major in Chemistry. In addition, a useful selection of Elective Courses should be made in order to augment the depth of the subject expected of a Major. ...
App. Chemistry
... Therefore the instructional days for the theory papers in a semester are 4 x 15(weeks)= 60 • There are 4 practicals (with 1 project) each of 3 hour duration for the 2 practical papers Total practical workload is 12 hours. Thus instructional days for the practical course of 4 practicals are 4 (practi ...
... Therefore the instructional days for the theory papers in a semester are 4 x 15(weeks)= 60 • There are 4 practicals (with 1 project) each of 3 hour duration for the 2 practical papers Total practical workload is 12 hours. Thus instructional days for the practical course of 4 practicals are 4 (practi ...
Print this article - Bangladesh Journals Online
... assignable for protons Hd and Ha respectively. The two doublets of doublet at δ 6.5 (JHa-Hb = JHb-Hc = J = 8.0 Hz) and 6.9 (JHb-Hc= JHc-Hd = J = 8.0 Hz) accounts for the Ha and Hd respectively, while the relatively downfield signal at δ 8.5 has been assigned for the imine (=N-H) proton of 2-mercapto ...
... assignable for protons Hd and Ha respectively. The two doublets of doublet at δ 6.5 (JHa-Hb = JHb-Hc = J = 8.0 Hz) and 6.9 (JHb-Hc= JHc-Hd = J = 8.0 Hz) accounts for the Ha and Hd respectively, while the relatively downfield signal at δ 8.5 has been assigned for the imine (=N-H) proton of 2-mercapto ...
TDB-5: Standards and conventions for TDB publications
... trihydroxyglutarate tris(2-pyridylmethyl)amine tetrakis(2-pyridylmethyl)amine 2,2’,2”-triaminotriethylamine triethylenetetraamine tryptophanate thiourea thyrosinate valinate ...
... trihydroxyglutarate tris(2-pyridylmethyl)amine tetrakis(2-pyridylmethyl)amine 2,2’,2”-triaminotriethylamine triethylenetetraamine tryptophanate thiourea thyrosinate valinate ...
Chemical Reactions and Stoichiometry
... examples of chemistry occurring before your eyes. When you heat a steak on a barbeque, the meat changes from a bright red colour to the brown colour that we recognize as a steak on our plate. What is occurring is that the proteins that compose the steak are being d enatured. In this particular exa ...
... examples of chemistry occurring before your eyes. When you heat a steak on a barbeque, the meat changes from a bright red colour to the brown colour that we recognize as a steak on our plate. What is occurring is that the proteins that compose the steak are being d enatured. In this particular exa ...
Document
... C) There will be a net gain in both product and reactant. D) There will be no net gain in either product or reactant. E) The equilibrium constant will decrease until it equals the reaction quotient. ...
... C) There will be a net gain in both product and reactant. D) There will be no net gain in either product or reactant. E) The equilibrium constant will decrease until it equals the reaction quotient. ...