Chemical Quantities and Aqueous Reactions
... In recent years scientists have become concerned because the amount of atmospheric carbon dioxide (CO2)—Earth’s most significant greenhouse gas in terms of its contribution to climate—is rising. More CO2 enhances the atmosphere’s ability to hold heat and may therefore lead to global warming, an incr ...
... In recent years scientists have become concerned because the amount of atmospheric carbon dioxide (CO2)—Earth’s most significant greenhouse gas in terms of its contribution to climate—is rising. More CO2 enhances the atmosphere’s ability to hold heat and may therefore lead to global warming, an incr ...
Document
... Heating value of a fuel • Lower heating value at constant pressure = symmetric of the standard enthalpy of combustion, per unit mass of fuel, when there is water vapour in the combustion products (always positive) • Lower heating value at constant volume = symmetric of the standard internal energy ...
... Heating value of a fuel • Lower heating value at constant pressure = symmetric of the standard enthalpy of combustion, per unit mass of fuel, when there is water vapour in the combustion products (always positive) • Lower heating value at constant volume = symmetric of the standard internal energy ...
Covert Chemical... 2_Couvertures English chimie 4
... Chemical Reactions 2: Equilibrium and Oxidation-reduction is the third of the three Learning Guides for the Secondary V Chemistry program, which comprises the following three courses: Gases Chemical Reactions 1: Energy and Chemical Dynamics Chemical Reactions 2: Equilibrium and Oxidation-reduction ...
... Chemical Reactions 2: Equilibrium and Oxidation-reduction is the third of the three Learning Guides for the Secondary V Chemistry program, which comprises the following three courses: Gases Chemical Reactions 1: Energy and Chemical Dynamics Chemical Reactions 2: Equilibrium and Oxidation-reduction ...
SQA Advanced Higher Chemistry Unit 2 Principles of Chemical
... Which of the following statements applies to this equation? 1. Calcium carbonate reacts with hydrochloric acid to produce calcium chloride solution, water and carbon dioxide. 2. One formula unit of calcium carbonate reacts with two formula units of hydrochloric acid to produce one formula unit each ...
... Which of the following statements applies to this equation? 1. Calcium carbonate reacts with hydrochloric acid to produce calcium chloride solution, water and carbon dioxide. 2. One formula unit of calcium carbonate reacts with two formula units of hydrochloric acid to produce one formula unit each ...
ChemConnections
... Spontaneous Processes • Thermodynamics is concerned with the question: can a reaction occur? • First Law of Thermodynamics: Energy is conserved. • Any process that occurs without outside intervention is spontaneous. • When two eggs are dropped they spontaneously break. • The reverse reaction (two e ...
... Spontaneous Processes • Thermodynamics is concerned with the question: can a reaction occur? • First Law of Thermodynamics: Energy is conserved. • Any process that occurs without outside intervention is spontaneous. • When two eggs are dropped they spontaneously break. • The reverse reaction (two e ...
AQA GCSE Chemistry My Revision Notes
... the production, the vapour of an alkane is passed over a hot catalyst to produce ethene. Ethene is then converted into ethanol. (h) Describe how ethanol, C2H5OH, can be produced from ethene. (2 marks) (i) Ethanol can be made using either sugar or alkanes as the starting material. Evaluate the advant ...
... the production, the vapour of an alkane is passed over a hot catalyst to produce ethene. Ethene is then converted into ethanol. (h) Describe how ethanol, C2H5OH, can be produced from ethene. (2 marks) (i) Ethanol can be made using either sugar or alkanes as the starting material. Evaluate the advant ...
electrical energy and capacitance
... What is the molecular formula of this compound? 1A. (1) C = 12.01 amu (2) H = 1.01 amu (3) C2 + H5 (4) C2H5 = 2(12.01 amu) + 5(1.01 amu) (5) EF = C2H5 = 29.07 g/mol (6) MF = 58.12 g/mol (7) MF = n(EF) (8) n = MF / EF (9) n = 58.12 / 29.07 (10) n = 2 (11) MF = (2)(C2H5) (12) MF = C4H10 MOLE TO MOLE C ...
... What is the molecular formula of this compound? 1A. (1) C = 12.01 amu (2) H = 1.01 amu (3) C2 + H5 (4) C2H5 = 2(12.01 amu) + 5(1.01 amu) (5) EF = C2H5 = 29.07 g/mol (6) MF = 58.12 g/mol (7) MF = n(EF) (8) n = MF / EF (9) n = 58.12 / 29.07 (10) n = 2 (11) MF = (2)(C2H5) (12) MF = C4H10 MOLE TO MOLE C ...
CLASSES AND NOMENCLATURE OF INORGANIC COMPOUNDS
... 9. Speed of what reactions increases if the 10. The law of mass action describes the temperature is increased? dependence of rate of chemical reaction on: A endothermic A the concentration of reactans B exothermic B areas of surface of clashing of reactive C anyone compounds D red-ox reaction C the ...
... 9. Speed of what reactions increases if the 10. The law of mass action describes the temperature is increased? dependence of rate of chemical reaction on: A endothermic A the concentration of reactans B exothermic B areas of surface of clashing of reactive C anyone compounds D red-ox reaction C the ...
Part A Completion
... Use this completion exercise to check your understanding of the concepts and terms that are introduced in this section. Each blank can be completed with a term, short phrase, or number. The oxidation number of an element in an uncombined state ...
... Use this completion exercise to check your understanding of the concepts and terms that are introduced in this section. Each blank can be completed with a term, short phrase, or number. The oxidation number of an element in an uncombined state ...
Ex - Bosna Sema
... the substance which is totally consumed when the chemical reaction is complete. The amount of product formed is limited by this reagent since the reaction cannot proceed further without it. The other reagents may be present in excess of the quantities required to react with the limiting reagent. 4 c ...
... the substance which is totally consumed when the chemical reaction is complete. The amount of product formed is limited by this reagent since the reaction cannot proceed further without it. The other reagents may be present in excess of the quantities required to react with the limiting reagent. 4 c ...
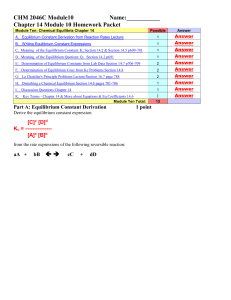
EQUILIBRIUM
... Thus the amount of solid NH4HS present does not affect the equilibrium. b) two points The equilibrium pressure of NH3 gas would decrease. In order for the pressure equilibrium constant, Kp, to remain constant, the equilibrium pressure of NH3 must decrease when the pressure of H2S is increased. Kp = ...
... Thus the amount of solid NH4HS present does not affect the equilibrium. b) two points The equilibrium pressure of NH3 gas would decrease. In order for the pressure equilibrium constant, Kp, to remain constant, the equilibrium pressure of NH3 must decrease when the pressure of H2S is increased. Kp = ...
EQUILIBRIUM
... Thus the amount of solid NH4HS present does not affect the equilibrium. b) two points The equilibrium pressure of NH3 gas would decrease. In order for the pressure equilibrium constant, Kp, to remain constant, the equilibrium pressure of NH3 must decrease when the pressure of H2S is increased. Kp = ...
... Thus the amount of solid NH4HS present does not affect the equilibrium. b) two points The equilibrium pressure of NH3 gas would decrease. In order for the pressure equilibrium constant, Kp, to remain constant, the equilibrium pressure of NH3 must decrease when the pressure of H2S is increased. Kp = ...
Photogeneration of Hydride Donors and Their Use Toward CO2
... Bis(2,2'-bipyridine)rhodium(I) from Temperature- and Pressure-Dependent Experimental and Theoretical Studies” Inorg. Chem. 2006, 45, 1595-1603. 2. Grills, D. C.; Huang, K.-W.; Muckerman, J. T.; Fujita, E. “Kinetic Studies of the Photoinduced Formation of Transition Metal-Dinitrogen Complexes Using T ...
... Bis(2,2'-bipyridine)rhodium(I) from Temperature- and Pressure-Dependent Experimental and Theoretical Studies” Inorg. Chem. 2006, 45, 1595-1603. 2. Grills, D. C.; Huang, K.-W.; Muckerman, J. T.; Fujita, E. “Kinetic Studies of the Photoinduced Formation of Transition Metal-Dinitrogen Complexes Using T ...
Coordination and Chemistry of Stable Cu (II) Complexes in the Gas
... where neutral metal-solvent complexes are prepared in a molecular beam, which is then ionized by high-energy electron impact. The success of this technique relies on the fact that multiply charged metal ions are generated after the metal has already been encapsulated within a stable solvent environm ...
... where neutral metal-solvent complexes are prepared in a molecular beam, which is then ionized by high-energy electron impact. The success of this technique relies on the fact that multiply charged metal ions are generated after the metal has already been encapsulated within a stable solvent environm ...
Chapter 19 Chemical Thermodynamics
... metal to the cooler water. The final temperature, after the metal and water achieve the same temperature (thermal equilibrium), will be somewhere between the initial temperatures of the metal and the water. (b) Experience tells us that this process is not spontaneous—we certainly have never seen hyd ...
... metal to the cooler water. The final temperature, after the metal and water achieve the same temperature (thermal equilibrium), will be somewhere between the initial temperatures of the metal and the water. (b) Experience tells us that this process is not spontaneous—we certainly have never seen hyd ...
Chapter 5
... 1970s, it has been found that many other transition metal complexes can undergo cyclocondensations: Ti,4 Zr, 5 Mo, 6 W, 7 Fe, 8 Ru, 9 Ni, 10 and very recently Rh 11 . Among these methods, the Pauson-Khand reaction stands out because of its experimental simplicity, functional group compatibility and ...
... 1970s, it has been found that many other transition metal complexes can undergo cyclocondensations: Ti,4 Zr, 5 Mo, 6 W, 7 Fe, 8 Ru, 9 Ni, 10 and very recently Rh 11 . Among these methods, the Pauson-Khand reaction stands out because of its experimental simplicity, functional group compatibility and ...