Chapter 1: Matter and Measurement
... Matter. Calculate H for the process in which 50.0 g of water is converted from liquid at 10.0°C to vapor at 25.0°C. Break the problem into two steps: Raise the temperature of the liquid first then completely vaporize it. The total enthalpy change is the sum of the changes in each step. ...
... Matter. Calculate H for the process in which 50.0 g of water is converted from liquid at 10.0°C to vapor at 25.0°C. Break the problem into two steps: Raise the temperature of the liquid first then completely vaporize it. The total enthalpy change is the sum of the changes in each step. ...
Document
... volumes of gas, at the same temperature and pressure contain the same number of particles. Moles are numbers of particles You can treat reactions as if they happen liters at a time, as long as you keep the temperature and pressure the same. 1 mole = 22.4 L @ STP ...
... volumes of gas, at the same temperature and pressure contain the same number of particles. Moles are numbers of particles You can treat reactions as if they happen liters at a time, as long as you keep the temperature and pressure the same. 1 mole = 22.4 L @ STP ...
Chemistry Higher Level Chapter 5 - Pearson Schools and FE Colleges
... All chemical reactions are accompanied by energy changes. Energy changes are vital. Our body’s processes are dependent on the energy changes which occur during respiration, when glucose reacts with oxygen. Modern lifestyles are dependent on the transfer of energy that occurs when fuels burn. As we e ...
... All chemical reactions are accompanied by energy changes. Energy changes are vital. Our body’s processes are dependent on the energy changes which occur during respiration, when glucose reacts with oxygen. Modern lifestyles are dependent on the transfer of energy that occurs when fuels burn. As we e ...
5 Energetics - Pearson Schools and FE Colleges
... All chemical reactions are accompanied by energy changes. Energy changes are vital. Our body’s processes are dependent on the energy changes which occur during respiration, when glucose reacts with oxygen. Modern lifestyles are dependent on the transfer of energy that occurs when fuels burn. As we e ...
... All chemical reactions are accompanied by energy changes. Energy changes are vital. Our body’s processes are dependent on the energy changes which occur during respiration, when glucose reacts with oxygen. Modern lifestyles are dependent on the transfer of energy that occurs when fuels burn. As we e ...
Topic 7b Redox notes
... hydrogen; reduction is the loss of oxygen or the gain of hydrogen. These definitions can only be used when a chemical reaction involves hydrogen and oxygen, and therefore their usefulness is limited. ...
... hydrogen; reduction is the loss of oxygen or the gain of hydrogen. These definitions can only be used when a chemical reaction involves hydrogen and oxygen, and therefore their usefulness is limited. ...
Chemistry - Sanskriti School
... Unit I: Some Basic Concepts of Chemistry General Introduction: Importance and scope of chemistry. Nature of matter, laws of chemical combination. Dalton’s atomic theory: concept of elements, atoms and molecules. Atomic and molecular masses. Mole concept and molar mass: percentage composition, empiri ...
... Unit I: Some Basic Concepts of Chemistry General Introduction: Importance and scope of chemistry. Nature of matter, laws of chemical combination. Dalton’s atomic theory: concept of elements, atoms and molecules. Atomic and molecular masses. Mole concept and molar mass: percentage composition, empiri ...
step by step Stoichiometry
... Or 80.3 divided by 55.847, multiplied by 3, divided by 2, multiplied by 28.01015 ...
... Or 80.3 divided by 55.847, multiplied by 3, divided by 2, multiplied by 28.01015 ...
Here`s - Sonlight
... memorize table 4.2. Example 4.3 gives you some practice in naming ionic compounds with 3transition metals. 4 ...
... memorize table 4.2. Example 4.3 gives you some practice in naming ionic compounds with 3transition metals. 4 ...
B.Sc Chemistry - Calicut University
... centered iv) develop the mental faculty of open mindedness and v) train students in the use of equipments in chemistry laboratories. The Higher Education Council of Kerala has taken the initiative to remodel the undergraduate syllabus by introducing the credit and semester system at this level also. ...
... centered iv) develop the mental faculty of open mindedness and v) train students in the use of equipments in chemistry laboratories. The Higher Education Council of Kerala has taken the initiative to remodel the undergraduate syllabus by introducing the credit and semester system at this level also. ...
4Chemical Quantities and Aqueous Reactions
... chemical reaction, the tomato sauce would be the limiting reactant, the reactant that limits the amount of product in a chemical reaction. Notice that the limiting reactant is simply the reactant that makes the least amount of product. Reactants that do not limit the amount of product—such as the cr ...
... chemical reaction, the tomato sauce would be the limiting reactant, the reactant that limits the amount of product in a chemical reaction. Notice that the limiting reactant is simply the reactant that makes the least amount of product. Reactants that do not limit the amount of product—such as the cr ...
Introductory Chemistry, 2nd Edition Nivaldo Tro
... number of molecules as well as the number of moles of each substance ...
... number of molecules as well as the number of moles of each substance ...
Chapter 20 Electrochemistry
... Cr2O72(aq) + 14 H+(aq) + 6 I(aq) 2 Cr3+(aq) + 3 I2(s) + 7 H2O(l) is spontaneous. A solution containing K2Cr2O7 and H2SO4 is poured into one beaker, and a solution of KI is poured into another. A salt bridge is used to join the beakers. A metallic conductor that will not react with either solutio ...
... Cr2O72(aq) + 14 H+(aq) + 6 I(aq) 2 Cr3+(aq) + 3 I2(s) + 7 H2O(l) is spontaneous. A solution containing K2Cr2O7 and H2SO4 is poured into one beaker, and a solution of KI is poured into another. A salt bridge is used to join the beakers. A metallic conductor that will not react with either solutio ...
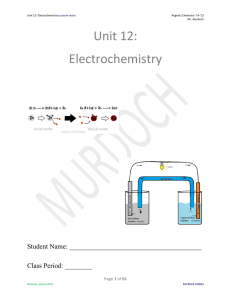
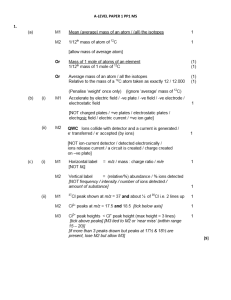
Unit 12: Electrochemistry
... yes it is, as I have taught Regents physics as well. But to understand what you can DO with electricity in physics, you need to understand how electricity is created in the chemical world. In today’s (2015) world, electricity and electronics dominate our lives. I typed this unit packet on an electro ...
... yes it is, as I have taught Regents physics as well. But to understand what you can DO with electricity in physics, you need to understand how electricity is created in the chemical world. In today’s (2015) world, electricity and electronics dominate our lives. I typed this unit packet on an electro ...
102MSJc14 - Louisiana Tech University
... completion, that is, until one of the reactants runs out. Many reactions do proceed essentially to completion: complete reactions are indicated by . For such reactions it can be assumed that the reactants are quantitatively converted to products and that the amount of limiting reactant that remains ...
... completion, that is, until one of the reactants runs out. Many reactions do proceed essentially to completion: complete reactions are indicated by . For such reactions it can be assumed that the reactants are quantitatively converted to products and that the amount of limiting reactant that remains ...
Document
... Do all reactants change into products during a reaction? Sometimes only a trace of reactants remains after the reaction is over. Figure 1 shows an example of such a reaction. Oxygen gas reacts with sulfur to form sulfur dioxide, as shown in the following chemical equation: S8(s) + 8O2(g) → 8SO2(g) ...
... Do all reactants change into products during a reaction? Sometimes only a trace of reactants remains after the reaction is over. Figure 1 shows an example of such a reaction. Oxygen gas reacts with sulfur to form sulfur dioxide, as shown in the following chemical equation: S8(s) + 8O2(g) → 8SO2(g) ...
Osmium(VIII) Catalyzed Oxidation of 6-Aminopenicillanic Acid
... which a known quantity of acrylonitrile (scavenger) had been added initially, was kept for 2 h in an inert atmosphere. On diluting the reaction mixture with methanol, a white precipitate was formed, indicating the intervention of free radicals in the reaction. The blank experiments of either DPC or ...
... which a known quantity of acrylonitrile (scavenger) had been added initially, was kept for 2 h in an inert atmosphere. On diluting the reaction mixture with methanol, a white precipitate was formed, indicating the intervention of free radicals in the reaction. The blank experiments of either DPC or ...
Thermodynamics ppt
... are pathway independent ⇒ since ∆ is defined as (final state – initial state) then ∆ anything can not depend on the states in between the initial and final states a.k.a. the “path” ⇒ if dealing with a state function you can use any path to solve for it ⇒ the easiest paths for ∆E and ∆H are… ∆E = qv ...
... are pathway independent ⇒ since ∆ is defined as (final state – initial state) then ∆ anything can not depend on the states in between the initial and final states a.k.a. the “path” ⇒ if dealing with a state function you can use any path to solve for it ⇒ the easiest paths for ∆E and ∆H are… ∆E = qv ...
chem 102 class notes - Louisiana Tech University
... completion, that is, until one of the reactants runs out. Many reactions do proceed . For such reactions essentially to completion: complete reactions are indicated by it can be assumed that the reactants are quantitatively converted to products and that the amount of limiting reactant that remains ...
... completion, that is, until one of the reactants runs out. Many reactions do proceed . For such reactions essentially to completion: complete reactions are indicated by it can be assumed that the reactants are quantitatively converted to products and that the amount of limiting reactant that remains ...