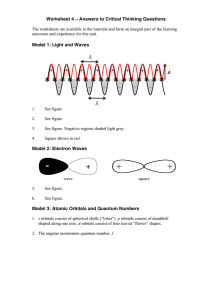
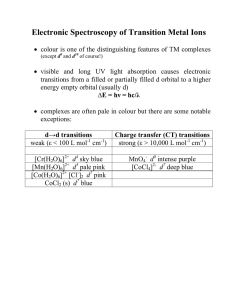
Answers to Critical Thinking Questions 4
... The 2s has one radial node and the 3s has two radial nodes. 3p have one radial node. In general, the number of radial nodes is equal to n – l - 1. ...
... The 2s has one radial node and the 3s has two radial nodes. 3p have one radial node. In general, the number of radial nodes is equal to n – l - 1. ...
LEP 5.1.08 Atomic spectra of two-electron systems: He, Hg
... 1. Determination of the wavelengths of the most intense spectral lines of He. 2. Determination of the wavelengths of the most intense spectral lines of Hg. Set-up and procedure The experimental set-up is shown in Fig. 1. Helium or mercury spectral tubes connected to the high voltage power supply uni ...
... 1. Determination of the wavelengths of the most intense spectral lines of He. 2. Determination of the wavelengths of the most intense spectral lines of Hg. Set-up and procedure The experimental set-up is shown in Fig. 1. Helium or mercury spectral tubes connected to the high voltage power supply uni ...
1. Select the correct statement about subatomic particles. a
... 21. Which element when combined with fluorine would most likely form an ionic compound? a. lithium c. phosphorus b. carbon d. chlorine 22. Compounds that are composed of ions ________. a. are molecular compounds b. have relatively high melting and boiling points c. are for the most part composed of ...
... 21. Which element when combined with fluorine would most likely form an ionic compound? a. lithium c. phosphorus b. carbon d. chlorine 22. Compounds that are composed of ions ________. a. are molecular compounds b. have relatively high melting and boiling points c. are for the most part composed of ...
Shell Structures and Level Statistics of a Quantum Dot
... The shell structures and the energy orderings are reflected in the addition energy spectrum. This is plotted as a function of N in Fig. 3. We remark the following features. In the absence of a magnetic field the addition energy alternate between a trough and a peak as a function of N . However, at N ...
... The shell structures and the energy orderings are reflected in the addition energy spectrum. This is plotted as a function of N in Fig. 3. We remark the following features. In the absence of a magnetic field the addition energy alternate between a trough and a peak as a function of N . However, at N ...
File
... Mechanical Energy Total mechanical energy of an object in motion is potential and kinetic energy combined. Mechanical energy = Joules = kg . m/s2 . m Mechanical energy = Potential energy + Kinetic energy ...
... Mechanical Energy Total mechanical energy of an object in motion is potential and kinetic energy combined. Mechanical energy = Joules = kg . m/s2 . m Mechanical energy = Potential energy + Kinetic energy ...
Evidence for Photons.wxp
... where + and , are arbitrary constants, and where 5 is the Boltzmann constant. This expression for the equation indeed gives Wein's displacement law and seemed to fit the existing experimental data (for low frequencies) reasonably well. EXERCISE: Beginning with Wein's expression for the spectral radi ...
... where + and , are arbitrary constants, and where 5 is the Boltzmann constant. This expression for the equation indeed gives Wein's displacement law and seemed to fit the existing experimental data (for low frequencies) reasonably well. EXERCISE: Beginning with Wein's expression for the spectral radi ...
simulation of insulating layers charging of nanomaterials under
... insulator. The semiconductor was a silicon substrate. The model structure of a nanoscale sample was a set of hexagonal nanocrystals, which were tight against each other with their faces [7]. A nanoparticle boundary was viewed as infinitely thin surfaces, and, while crossing these surfaces, an electr ...
... insulator. The semiconductor was a silicon substrate. The model structure of a nanoscale sample was a set of hexagonal nanocrystals, which were tight against each other with their faces [7]. A nanoparticle boundary was viewed as infinitely thin surfaces, and, while crossing these surfaces, an electr ...
Chapter 9 - Fayetteville State University
... -212) Ionic Bond: The interaction between the atoms is due to transference of electrons from atom to the other, which cause a strong interaction between the ions formed. 13) Atoms Groups: atoms can interact between them to form stable positive of negative ions, examples are the sulfate and the nitr ...
... -212) Ionic Bond: The interaction between the atoms is due to transference of electrons from atom to the other, which cause a strong interaction between the ions formed. 13) Atoms Groups: atoms can interact between them to form stable positive of negative ions, examples are the sulfate and the nitr ...
Name: Date: Period: Who is the Father of Atomic Theory? What
... 3. How many electrons can exist on the following energy levels? 2nd: 3rd: 4th: 4. What characteristic of an atom tells us how many protons reside in the nucleus and helps to indentify the element? 5. Fill in the blanks. – “All atoms with the number of are of the element.” 6. What do we call two atom ...
... 3. How many electrons can exist on the following energy levels? 2nd: 3rd: 4th: 4. What characteristic of an atom tells us how many protons reside in the nucleus and helps to indentify the element? 5. Fill in the blanks. – “All atoms with the number of are of the element.” 6. What do we call two atom ...
Chemical Terms and Keywords
... This review sheet contains an alphabetical list of chemical terms, keywords, and equations used or discussed in Chemistry 130. For each term or keyword, you should be able to write a few sentences about the topic and its relationships to other topics in the same area. For each equation, you should b ...
... This review sheet contains an alphabetical list of chemical terms, keywords, and equations used or discussed in Chemistry 130. For each term or keyword, you should be able to write a few sentences about the topic and its relationships to other topics in the same area. For each equation, you should b ...
wave function - Purdue Physics
... • The experiment also shows aspects of particle-like behavior since the electrons arrive one at a time at the screen, and also don’t just go in straight lines. • In principle, you can slowly build up an interference pattern over time even if only one electron (or photon) per second arrives at the sc ...
... • The experiment also shows aspects of particle-like behavior since the electrons arrive one at a time at the screen, and also don’t just go in straight lines. • In principle, you can slowly build up an interference pattern over time even if only one electron (or photon) per second arrives at the sc ...
schoa - Schieck
... 9. When creating his new atomic theory, Bohr used on important new idea (theoryP and primarily one important experimental area of study. Identify each. 10. State two differences between Excitation and Relaxation 11. What is the empirical (observed) distinction between emission and absorption spectra ...
... 9. When creating his new atomic theory, Bohr used on important new idea (theoryP and primarily one important experimental area of study. Identify each. 10. State two differences between Excitation and Relaxation 11. What is the empirical (observed) distinction between emission and absorption spectra ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.